- Title
-
Vegfa signaling promotes zebrafish intestinal vasculature development through endothelial cell migration from the posterior cardinal vein
- Authors
- Koenig, A.L., Baltrunaite, K., Bower, N.I., Rossi, A., Stainier, D.Y., Hogan, B.M., Sumanas, S.
- Source
- Full text @ Dev. Biol.
|
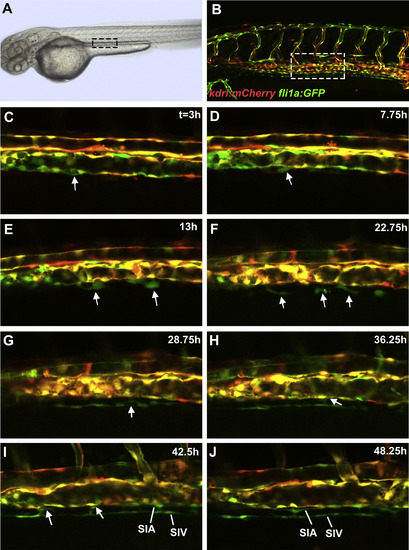
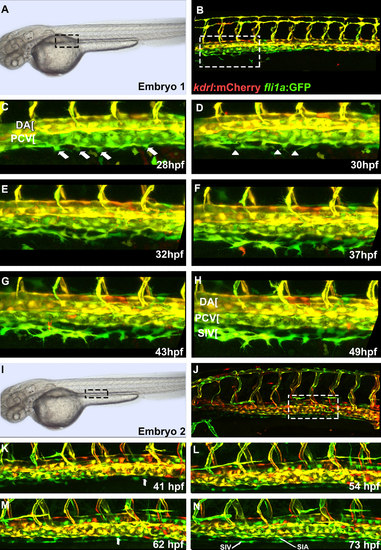
Cells from the posterior cardinal vein form the sub-intestinal vein, and the supra-intestinal artery. Confocal microscope timelapse images of live Tg(kdrl:mCherry);Tg(fli1a:EGFP) embryos beginning at approximately 25 hpf. (A and B) indicate region of interest displayed in other panels. Note that subintestinal vessel progenitors have very low kdrl:mCherry expression and appear green while endothelial cells within the axial vessels show both mCherry and GFP expression. (C) A thickened cell within the ventral wall of the PCV is observed which delaminates in (D) (arrows). Note the large heterogeneity of transgene expression within the wall of the PCV with mCherry negative cells interspersed next to mCherry positive cells. (E) Multiple SIV progenitors are observed (arrows) which still make contacts with the PCV. (F) SIV progenitors (arrows) acquire elongated shape with some of them still contacting the PCV. (G) SIV progenitors start to coalesce into a primordial vessel. Some still make contacts with the PCV (arrow). (H) SIV progenitors have largely coalesced into a vessel. Notice a new cell emerging from the PCV (arrow). (I) Multiple cells emerge from the PCV (arrows) and start coalescing into the SIA. Filopodia bridging the SIA and SIV are apparent. (J) Separate SIA and SIV primordial vessels are apparent. Anterior-left, dorsal-top. |
|
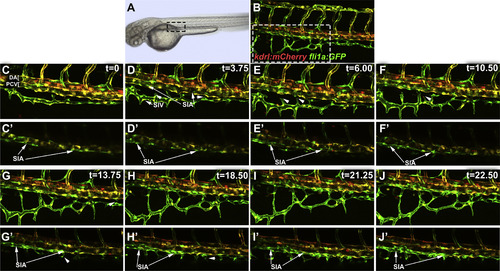
Coalescence of angioblasts into the supra-intestinal artery and interconnecting vessel formation. Confocal microscope timelapse images of live Tg(kdrl:mCherry);Tg(fli1a:EGFP) embryos beginning at approximately 56 hpf. Note that subintestinal vessel progenitors have low mCherry expression and appear mostly green. (A and B) indicate region of interest displayed in other panels. (C-J) Maximum intensity projections of confocal images at selected time points. (C′-J′) Selected confocal sections were combined in a maximum intensity projection of the same region as in C-J to more clearly visualize SIA. At 56 hpf, portions of the SIA are already visible with few connections to the SIV (C,C′). Over a period of 24 h (D-J′), cells from the PCV contribute to the further development of the SIA while branches from the SIV, indicated by arrowheads, extend toward the SIA and form interconnecting vessels (ICVs). Anterior-left, dorsal-top. |
|
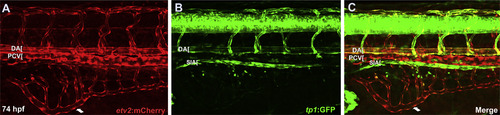
SIA but not SIV displays arterial specific Notch reporter tp1:GFP expression. Confocal microscope images of Tg(etv2:mCherry);Tg(tp1:GFP) embryos. (A-C) GFP expression indicating Notch responsive cells is restricted to the DA, arterial ISVs, and the SIA at 74 hpf. |
|
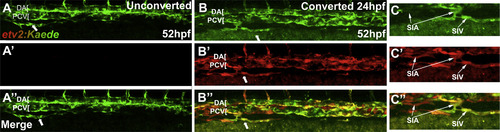
Cells in the sub-intestinal vein are derived from existing vasculature. Confocal microscope images of live Tg(etv2:Kaede) embryos following photoconversion. Note that etv2:Kaede line exhibits mosaic expression and not all vascular endothelial cells are labeled. (A-A′′) Unconverted control demonstrates that only converted cells are visible in the red channel. (B-B′′) Photoconversion at 24 hpf prior to any formation of intestinal vasculature results in all Kaede-positive cells in the SIV being marked by the converted (red) form of Kaede. This argues that SIV progenitors are derived from existing endothelial cells already expressing etv2:Kaede in the embryo at 24 hpf. (C-C′′) Cropped regions of converted embryos demonstrate that both the SIA and SIV are visible in the red channel. Arrows indicate SIV. Anterior-left, dorsal-top. |
|
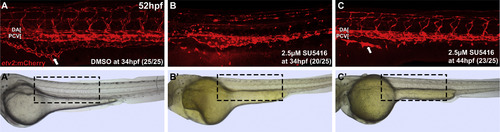
Vegf signaling is necessary for intestinal vascularization. Confocal (A-C) and brightfield images (A′-C′) of live Tg(etv2:mCherry) embryos at 52 hpf. (B) 2.5 µM SU5416 treatment from 32 to 52 hpf results in near complete loss of SIV as well as nearly complete regression of ISVs as compared to the DMSO treated control (A), while milder defects are observed in both ISVs and SIV in embryos treated from 44-52 hpf (C). Arrows indicate fully formed (A) or fragmented SIV (B and C). Treated embryos display pericardial edema but otherwise are morphologically normal (A′-C′). Anterior-left, dorsal-top. Boxed region in brightfield images demonstrates area shown by confocal imaging. |
|
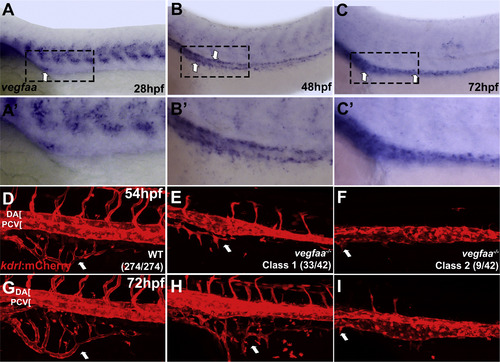
vegfaa provides a candidate guidance signal required for intestinal vessel development. In situ hybridization of wild type embryos (A-C′) and confocal images of live Tg(kdrl:mCherry) embryos (D-F′). (A-C) vegfaa is expressed at 28, 48, and 72 hpf in a narrow stripe of cells within the endoderm along the yolk and yolk extension in the same region where angioblasts coalesce into the SIA and SIV. (A′-C′) show higher magnification of the embryos in A-C. (D-F) vegfaa-/- embryos at 54 hpf exhibit strong inhibition (classs 1) or complete absence of SIV development (class 2). In addition, single axial vessel and inhibition of intersegmental vessel angiogenesis, where ISVs are absent or do not extend past the midline, are observed. (D′-F′) The same embryos at 72 hpf show little to no recovery of more severe defects. (Class 1 - moderate SIV defect, Class 2 - absent or nearly absent SIV). Arrows indicate SIV. Anterior-left, dorsal-top. |
|
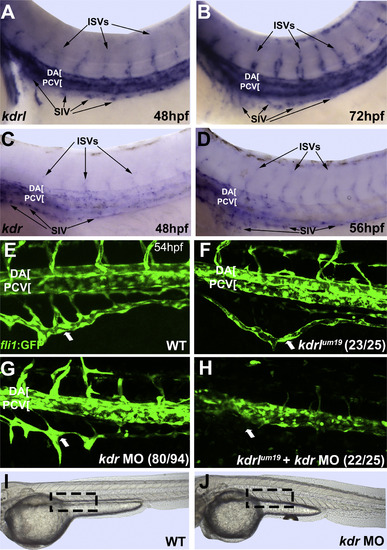
Vegfr2 homologs Kdr and Kdrl function redundantly in SIV development. (A-D) In situ hybridization analysis of kdrl and kdr expression in the SIV progenitors (arrows) in wild-type embryos at 48-72 hpf. (E-H) As analyzed by confocal imaging of live fli1a:GFP embryos, kdrl-/- mutants (F) display an underdeveloped and mildly mispatterned SIV that lacks connections to the SIA and form only partially extended intersegmental vessels, while kdr morphants (G) also demonstrate mispatterned and underdeveloped ISVs and partial SIV development, but retain connections to the SIA. Combined kdrl-/-, kdr morphant embryos (H) display more severe axial vessel, ISV, and SIV defects than the kdrl-/- alone, resembling vegfaa-/- embryos. (I and J) As shown by brightfield imaging of live embryos, kdr morphants display a slight curvature of the body axis (J), but are otherwise phenotypically normal compared to uninjected controls (I). Large arrows indicate SIV. Anterior-left, dorsal-top. |
|
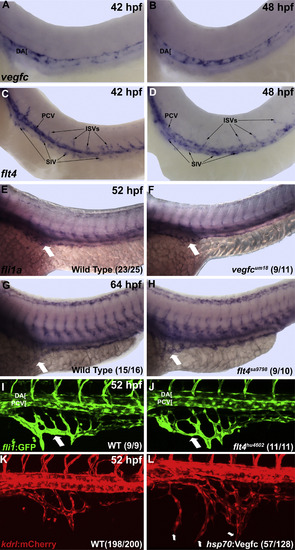
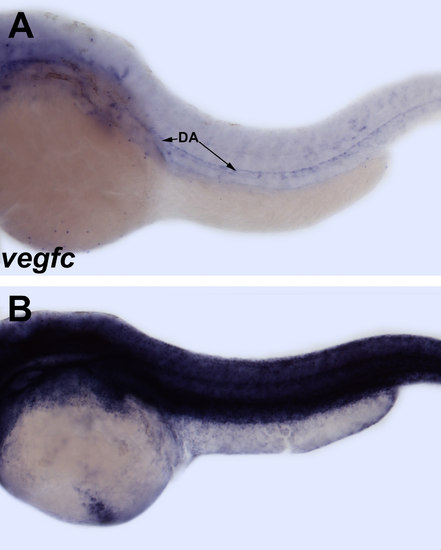
vegfc and flt4 are dispensable for formation, but sufficient to induce ectopic growth of the SIV. (A and B) Based on in situ hybridization, vegfc expression is localized to the dorsal aorta, while flt4 shows expression in the progenitors of the intestinal vessels at 42 and 48 hpf (C and D). (E-H) In situ hybridization of vegfc-/-, flt4-/-, and sibling embryos for fli1a. (E and F) vegfc-/- embryos demonstrate normal SIV development at 52 hpf as compared to wild type and heterozygous siblings. Homozygous flt4sa9798 (G and H) and flt4hu4602 (I and J) embryos also have normal SIV development at 64 hpf and 52 hpf by in situ hybridization (G and H) and confocal microscopy of Tg(fli1a:GFP) embryos (I and J) as compared to wild-type and heterozygous siblings. (K and L) Heat shock induced overexpression of vegfc in hsp70: Vegfc embryos at 30 hpf results in hyperbranching and ectopic ventral sprouting of the SIV at 52 hpf as observed by confocal imaging of Tg(kdrl:mCherry). Large arrows indicate SIV. Anterior-left, dorsal-top. |
|
Intestinal vasculature forms through endothelial cell migration from the posterior cardinal vein. Confocal microscope images of live Tg(kdrl:mCherry);Tg(fli1a:EGFP) embryos at 28-48 hpf. (A,B) regions shown in panels C-H are indicated by dashed boxes. (C-H) Endothelial cells begin to migrate out of the posterior cardinal vein at 28 hpf and by 35 hpf begin to coalesce to form the SIV (anterior portion of SIV). (I,J) regions shown in panels K-N are indicated by dashed boxes. (K-N) Cropped image of posterior region of the trunk in another embryo shows cells migrating from the posterior cardinal vein that form both the SIV (K) and SIA (M) as well as connections that form between SIV and SIA. Arrows indicate cells migrating from PCV while arrowheads mark gaps that are closing by extending filopodia. Anterior-left, dorsal-top. |
|
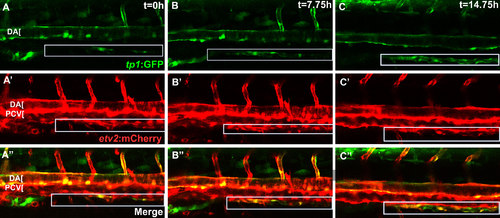
Upregulation of arterial Notch reporter tp1:GFP expression in SIA progenitors. Confocal microscope timelapse images of Tg(etv2:mCherry);Tg(tp1:GFP) embryos beginning at approximately 60 hpf. (A-C) Endothelial progenitors in the SIA express etv2:mCherry as they migrate from the PCV, but arterial specifc Notch reporter tp1:GFP expression is gradually upregulated as SIA progenitors coalesce into a blood vessel. Box indicates SIA. |
|
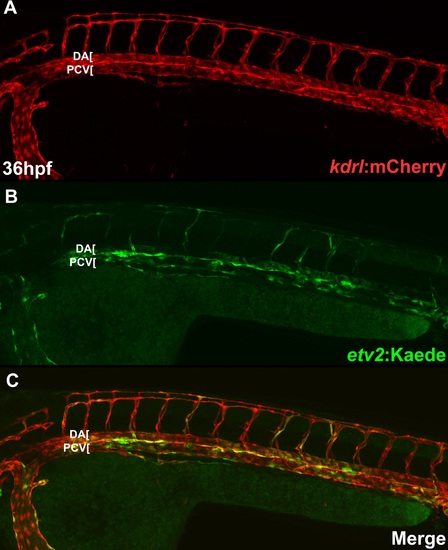
etv2:Kaede overlaps with vascular endothelial kdrl:mCherry expression. Confocal microscope imaging of Tg(kdrl:mCherry);Tg(etv2:Kaede) embryos at 36 hpf. |
|
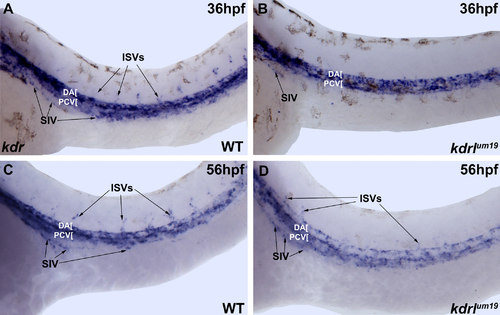
kdr expression is not significantly affected in kdrlum19 embryos. Brightfield microscope images of in situ hybridization staining. kdr is expressed at similar levels in WT (A,C) and kdrlum19 (B,D) embryos at 36 and 56 hpf including the developing SIV (indicated by arrows). |
|
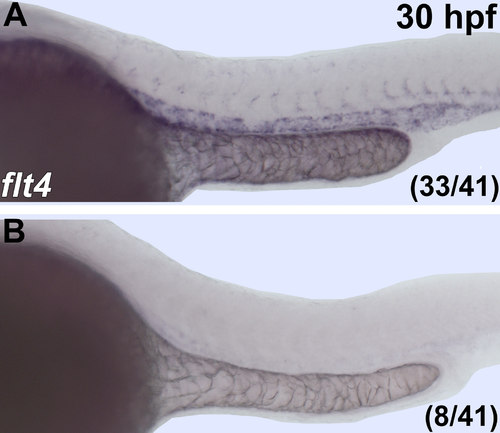
flt4 mRNA is absent or greatly reduced in flt4sa9798 mutants. in situ hybridization of flt4 with 30hpf embryos from an incross of heterozygous flt4sa9798 carriers demonstrate that 19.5% of embryos lack flt4 RNA signal. Anterior-left, dorsal-top. |
|
60 minute heat shock results in global overexpression of vegfc in hsp70:Vegfc embryos. Brightfield microscope images of in situ hybridization staining for vegfc. 60 minute heat shock at 32 hpf results in ectopic vegfc expression in the entire embryo at 36 hpf (B) compared to non-heat shocked transgenic embryos at the same stage (A). |
|
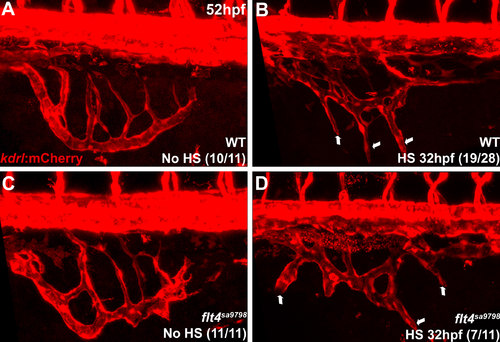
Overexpression of vegfc induces ectopic branching of intestinal vasculature in flt4 mutants. Confocal microscope images of Tg(kdrl:mCherry) embryos. 60 minute heat shock induction of vegfc in Tg(hsp70:vegfc) embryos at 32 hpf results in hyperbranching and ectopic sprouting from the SIV (B,D) indicated by arrows, compared to control non-heat shocked embryos (A,C) at 52 hpf. Embryos were individually genotyped for flt4 mutation after imaging. |
Reprinted from Developmental Biology, 411(1), Koenig, A.L., Baltrunaite, K., Bower, N.I., Rossi, A., Stainier, D.Y., Hogan, B.M., Sumanas, S., Vegfa signaling promotes zebrafish intestinal vasculature development through endothelial cell migration from the posterior cardinal vein, 115-27, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.