- Title
-
Myocardium and BMP signaling are required for endocardial differentiation
- Authors
- Palencia-Desai, S., Rost, M.S., Schumacher, J.A., Ton, Q.V., Craig, M.P., Baltrunaite, K., Koenig, A.L., Wang, J., Poss, K.D., Chi, N.C., Stainier, D.Y., Sumanas, S.
- Source
- Full text @ Development
|
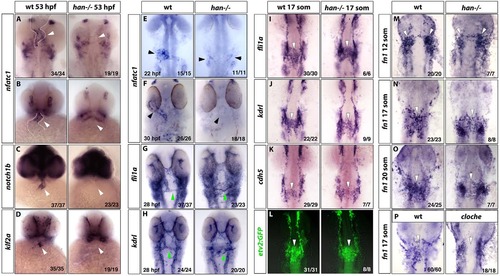
Endocardium fails to differentiate in hand2 mutants. (A-D) In situ hybridization staining for nfatc1 (A, flat-mount embryos; B, ventral view of whole-mount embryos), notch1b (C) and klf2a (D) in wild-type siblings (left column) and hand2 mutants (right column) at 53hpf shows the loss of differentiated endocardial markers in hand2 mutants. White arrowheads denote the endocardium, which is also outlined by a white dotted line (A,B). Expression of these endocardial markers becomes enriched at the atrio-ventricular (AVC) boundary at this stage, although some expression throughout the endocardium is still apparent. (E,F) Endocardial nfatc1 expression (arrowheads) is greatly reduced and split bilaterally (E) or absent (F) in hand2 mutants at 22hpf (E) and 30hpf (F), as analyzed by in situ hybridization. (G,H) Expression of vascular endothelial/endocardial markers fli1a (G) and kdrl (H) at 28hpf. The endocardial precursors form an endocardial sheet at the midline region but fail to coalesce into a cardiac cone. A subset of these progenitors contributes to the first aortic arch, which appears to form normally in hand2 mutants (green arrowheads). (I-L) Expression of fli1a, kdrl, cdh5 and etv2:GFP in the midline population of vascular endothelial/endocardial precursor cells (arrowheads) is largely unaffected in hand2 mutants at the 17-somite stage. (M-O) Fibronectin (fn1) expression analysis in hand2 mutants. fn1 expression shows no difference in premigratory endocardial precursors (white arrowheads) between wild type (left column) and hand2 mutant (right column) embryos at the 12-somite stage (M). At the 17-somite (N) and 20-somite (O) stages, hand2 mutants have fewer fn1-expressing endocardial cells at the midline compared with wild-type embryos, while many bilaterally located cells are observed. (P) cloche mutants do not show fn1 expression at the midline at the 17-somite stage, which suggests that the midline population of fn1-expressing cells corresponds to the endothelial/endocardial progenitors. All panels were flatmounted ventral side upwards, except for the ventral views in whole-mounted embryos in B-D. The number of embryos with the representative expression pattern out of the total number of embryos analyzed is shown in the lower right-hand corner. |
|
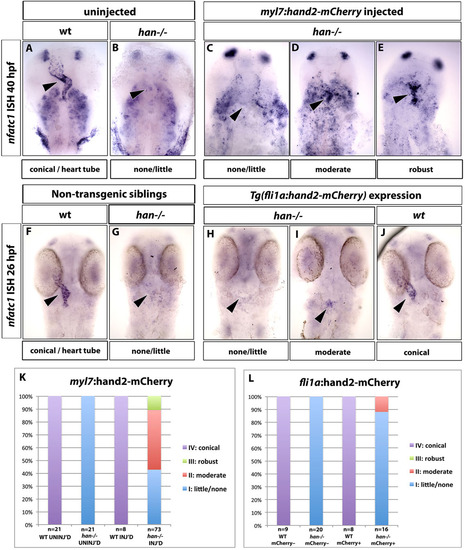
Hand2 function is required within the myocardium for endocardial nfatc1 expression. (A-E) Transient overexpression of a hand2-2A-mCherry construct under the myocardial-specific myl7 promoter results in a partial rescue of nfatc1 expression in hand2 mutants. In situ hybridization analysis of nfatc1 expression at 40hpf. In uninjected wild-type embryos, nfatc1 is expressed throughout the endocardium with a higher concentration at the A/V boundary (A, arrowhead). Endocardial nfatc1 expression is absent in uninjected hand2 mutants (B, arrowhead). (C-E) Representative embryos within different categories of rescued endocardial nfatc1 expression (arrowheads). nfatc1 expression is also observed in pharyngeal arches. (F-J) Endothelial/endocardial-specific overexpression of hand2-2A-mCherry under the fli1a promoter fails to rescue endocardial nfatc1 expression in hand2 mutants, as analyzed by in situ hybridization at 26hpf. (F,G) Non-transgenic (fli1a:hand2-mcherry) embryos in wild-type (F) and hand2-/- (G) background. (H-J) fli1a:hand2-mCherry-positive embryos in wild-type (J) and hand2-/- (H,I) background. Eighty-nine percent of fli1a:hand2-mCherry+; hand2-/- embryos had little or no nfatc1 expression (H) and 11% had moderate nfatc1 expression, not significantly different from non-transgenic (fli1a:hand2-mCherry) embryos (see L). Arrowheads indicate endocardial nfatc1 expression. (K,L) Percentages of myl7:hand2-mCherry and fli1a:hand2-mCherry embryos and their siblings in wild-type and hand2-/- background that showed different levels of nfatc1 expression. |
|
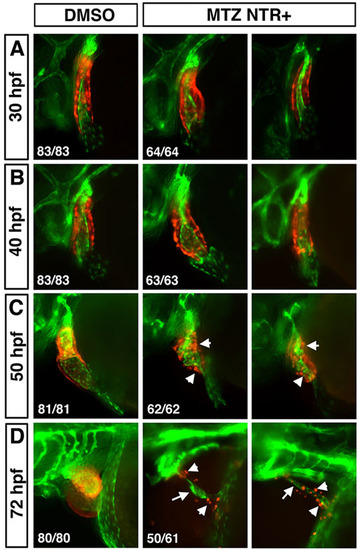
Myocardium is not necessary for the survival of endocardial cells. Myocardium or endocardium are observed in live myl7:mCherry-NTR; kdrl:GFP transgenic embryos incubated in either 1% DMSO (left column) or 10mM MTZ solution, respectively (middle and right columns show examples of two representative embryos for each stage). mCherry-expressing myocardial cells are scattered and disorganized at 50hpf (C, arrowheads) and only a few mCherry+ cells remain at 72hpf (D, middle and right columns, arrowheads). Endocardium is distorted but present in C,D (arrows). |
|
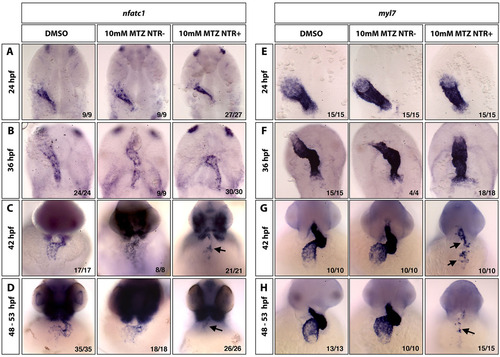
Nfatc1 expression within the endocardium is lost in the absence of myocardial cells. In situ hybridization analysis for endocardial nfatc1 expression (A-D) and myocardial myl7 expression (E-H) in control 1% DMSO-treated embryos (left column), control myl7:mCherry-NTR-negative embryos treated with 10mM MTZ (middle column) and myl7:mCherry-NTR+ embryos treated with 10mM MTZ (right column). Loss of myocardial myl7 expression is observed in G,H (right column, arrows), which correlates with the loss of nfatc1 expression (C,D; right column, arrows). (A,B,E,F) Flat-mount embryos, ventral view; (C,D,G,H) whole-mount embryos, ventral view of the heart region. |
|
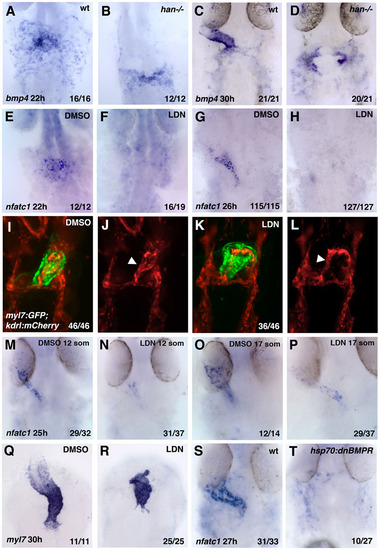
BMP signaling is required for endocardial differentiation. (A-D) As analyzed by in situ hybridization, myocardial bmp4 expression is greatly reduced in hand2-/- embryos (B,D) when compared with their siblings at 22hpf (A,B) and 30hpf (C,D). (E-H) Endocardial nfatc1 is greatly downregulated in BMP inhibitor LDN193189-treated embryos at 22hpf (E,F) and 26hpf (G,H) when compared with 0.1% DMSO-treated controls. (I-L) mCherry and GFP fluorescence are observed in the endocardium and myocardium, respectively, in 24 hpf fixed myl7:GFP; kdrl:mCherry embryos that were treated with 10µM LDN193189 from the the tailbud stage onwards (I,K, merged channels; J,L, mCherry channel only). Arrowheads indicate endocardial tube. (M-P) Treatment of embryos with BMP inhibitor LDN193189 starting at the 12- and 17-somite stages results in significant inhibition of nfatc1 expression at 26-28hpf, as analyzed by in situ hybridization. Treatment starting at the 12-somite stage resulted in more significant downregulation of nfatc1 expression than the treatment starting at the 17-somite stage. (Q,R) Myocardial myl7 expression at 30hpf is dysmorphic but does not show significant downregulation in the embryos treated with LDN193189 starting at the tailbud stage. (S,T) Heat-shock-inducible expression of DNBMPR-GFP results in significant downregulation of nfatc1 expression. hsp70:dnBMPR-positive embryos and their siblings were heat-shocked at the tailbud stage for 60min and analyzed at 27hpf. |
|
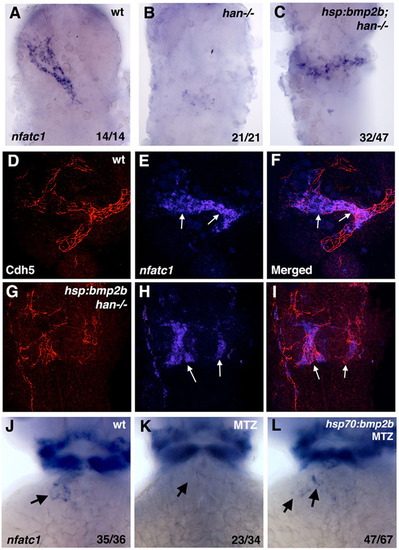
Heat-shock inducible Bmp2b expression partially rescues nfatc1 expression in hand2 mutants and MTZ-treated embryos. (A-C) Endocardial nfatc1 expression is restored in heat-shocked hand2-/-; hsp70:bmp2b transgenic embryos (C) when compared with hand2-/- embryos (B) at 30hpf. Ubiquitous bmp2b induction does not rescue the shape of the endocardial cone. The embryos were obtained by crossing hsp70:bmp2b+/?; hand2+/-×hand2+/- carriers in myl7:GFP background, where a mixture of heterozygous and homozygous hsp70:bmp2b adults were used; therefore, 50-100% of the embryos have hsp70:bmp2b transgene. hand2-/- embryos were identified based on the bilateral myl7:GFP expression. (D-I) hsp70:bmp2b induces nfatc1 expression in hand2 mutants in the endothelial/endocardial cells. Confocal maximum intensity projections of flat-mounted embryos (ventral views) analyzed by fluorescent in situ hybridization/immunohistochemistry for nfatc1 expression (in situ hybridization, blue, E,F,H,I) and Cdh5/VE-Cadherin (immunofluorescence, red, D,F,G,I) at 24hpf. (F,I) Merged channels. (D-F) Wild-type embryo; (G-I) hand2-/-; hsp70:bmp2b embryo. nfatc1 is expressed in the endocardial tube in E,F (arrows), whereas Cdh5 staining outlines the surfaces of endocardial cells, as well as endothelial cells of blood vessels. Patches of mislocalized nfatc1 expression are present in hand2-/-; hsp70:bmp2b embryos (arrows, H,I), which overlap with Cdh5 staining, confirming their endocardial identity. (J-L) Partial rescue of nfatc1 expression at 46hpf stage in heat-shocked myl7:mCherry-NTR; hsp70:bmp2b transgenic embryos treated with MTZ to deplete the myocardium. nfatc1 expression is absent in the MTZ-treated myl7:mCherry-NTR embryo (arrow, K), and partially restored in a heat-shocked myl7:mCherry-NTR; hsp70:bmp2b embryo (arrows, L). Heat-shock or MTZ treatment alone did not affect nfatc1 expression (not shown). |
|
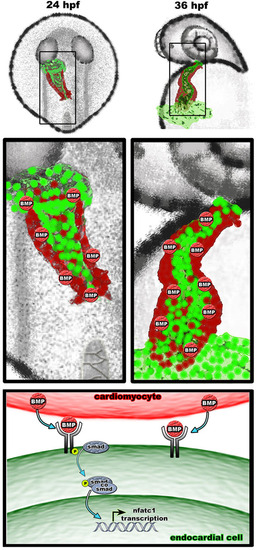
A proposed model for myocardial-derived BMP signaling during the initiation of nfatc1 transcription and endocardial differentiation. Myocardium secretes BMP homologs which induce transcription of nfatc1 and other target genes in the adjacent vascular endothelium at 22-24 hpf, thus establishing endocardial identity of these cells. Continued myocardial signaling is required for the maintenance of endocardial identity at 36 hpf and later stages. |
|
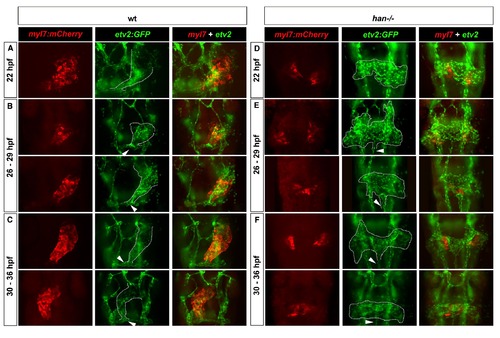
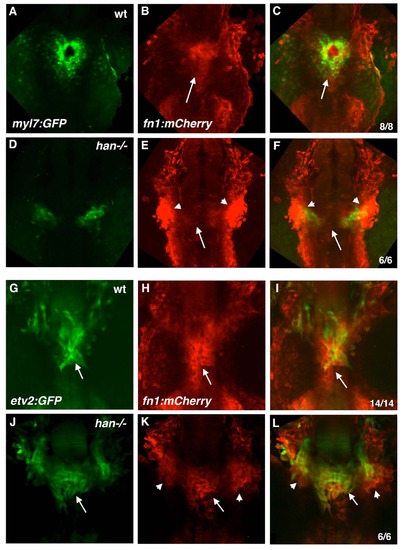
Loss of Hand2 function prevents endocardial cone morphogenesis and causes aberrant midline migration of etv2:GFP cells. (A-C) Double transgenic hearts labeled red for myocardium (myl7:mCherry, left columns) and green for endothelial/endocardial cells (etv2:GFP, middle columns) in wild-type (wt, A-C) and han-/- (D-F) embryos at 22 hpf (A and D), 26-29 hpf (B and E), and 30-36 hpf (C and F). All panels are fluorescent images of GFP and mCherry fluorescence in fixed and flatmounted embryos, ventral views. Dotted outlines indicate endocardial cones (A-C) or presumptive endocardial precursors (D-F). Note the inflow tract connecting to the first aortic arch in wt embryos (arrowheads, A-C) and ectopic vessel extending posteriorly from the endocardial sheet in han-/- mutants (arrowheads, D-F). |
|
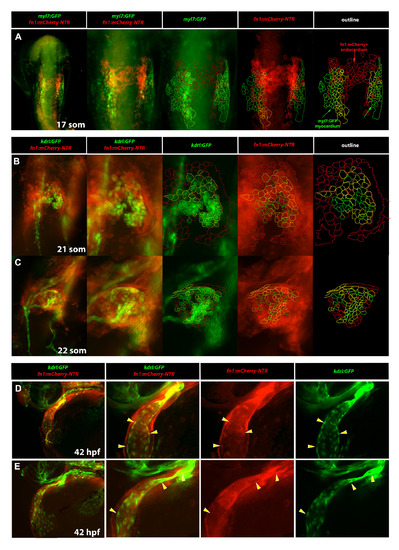
Fibronectin expression dynamically labels both endocardial and myocardial cells. (A) Analysis of fn1:mCherry-NTR; myl7:GFP expression at the 17-somite stage. Note that there is a partial overlap between the bilaterally located fn1:mCherry-NTR and myocardial myl7:GFP expression while mCherry-positive cells present at the midline correspond to the presumptive endocardial progenitors and are GFP-negative. (B,C) kdrl:GFP; fn1:mCherry-NTR expression in the cardiac region at the 21-somite (B) and 22-somite (C) stages. Note that in (B) the cardiac plate is arranged with kdrl:GFP labeled endocardial cells in the middle (B, 3rd & 5th column, green outline) and fn1:mCherry-NTR labeled myocardial cells (B, 4th & 5th column, red outline) on the outer periphery of the cardiac plate. Endocardial cells co-expressing both kdrl:GFP and fn1:mCherrry-NTR (B, 5th column, yellow outline) are generally located at the interface/border between endocardium and myocardium. At the 22-somite stage, the cardiac plate rearranges to begin cardiac cone extension (C). During this complex cellular rearrangement, yellow endocardial cells co-expressing both kdrl:GFP and fn1:mCherry-NTR (C, 5th column) are distributed throughout the growing cone. (D,E) kdrl:GFP; fn1:mCherry-NTR expression in the cardiac region at 42 hpf. Higher magnification view of the ventricle and outflow tract is shown in (E). Note that fn1:mCherry-NTR expression is more robust in the myocardial layer than in the endocardial layer, especially at the ventricular pole. However, there is a subtle but present expression of fn1:mCherry-NTR (3rd column) in the inner endocardial layer (4th column) of the heart (yellow arrowheads). (A-C) are whole embryo, ventral flat mount views. (D,E) are whole embryo, anterolateral views. All panels show GFP and mCherry fluorescence in fixed embryos. |
|
fn1:mCherry-NTR expression in han mutants and their wild-type siblings. Confocal microscopy images of live myl7:GFP; fn1:mCherry-NTR (A-F) and etv2:GFP; fn1:mCherry-NTR (G-L) transgenic embryos at the 22-24-somite stages. Maximum intensity projections of GFP (A,D,G,J), mCherry (B,E,H,K) and merged channels (C,F,I,L) are shown. Note that the myocardial myl7:GFP expression has only limited overlap with the endocardial fn1:mCherry expression at the midline (arrow, B,C) in wild-type (wt) embryos, while endocardial fn1:mCherry expression is reduced in han mutants (arrow, E,F), which exhibit bilateral domains of intense fn1:mCherry expression (arrowheads, E,F). Endocardial etv2:GFP expression largely overlaps with the midline domain of fn1:mCherry expression (arrows G-I) in wt embryos, while han mutants exhibit bilateral fn1:mCherry expression domains (arrowheads, K,L) which significantly overlap with etv2:GFP expression. Dorsal views, anterior is to the top. |
|
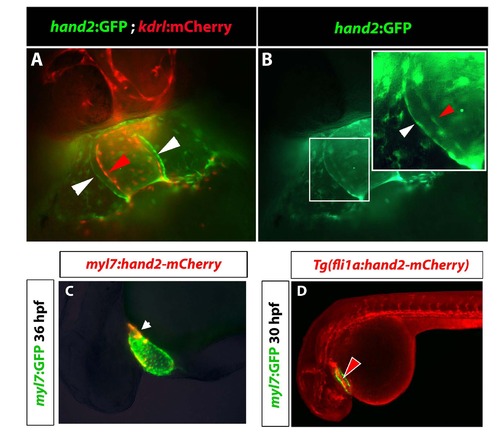
Hand2:GFP, myl7:hand2-mCherry and fli1a:hand2-mCherry reporter expression. (A,B) Fluorescent imaging of cardiac region in hand2:GFP; kdrl:mCherry transgenic embryos at 35 hpf. (A) kdrl:mCherry+ endocardial cells are shown in red and hand2:GFP+ myocardial cells in green. (B) GFP channel shows both myocardial (white arrowhead) and endocardial cells (red arrowhead) labeled with hand2:GFP. (C) Mosaic expression of injected myl7:hand2-mCherry construct overlaps with Tg(myl7:GFP) fluorescence at 36 hpf. Fluorescence in lightly fixed embryos is shown. (D) mCherry fluorescence is observed in the vasculature and the endocardium at 30 hpf in lightly fixed Tg(fli1a:hand2-mCherry); Tg(myl7:GFP) embryos. |
|
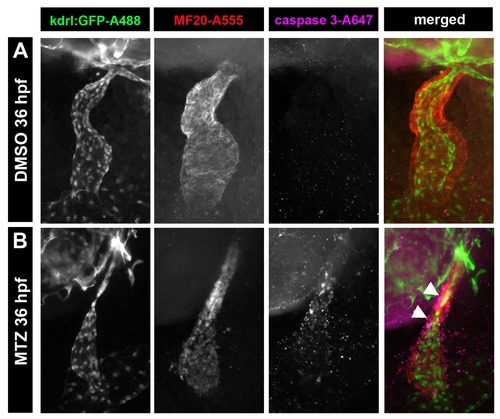
MTZ treatment induced apoptosis specific to myocardial cells in myl7:mCherry-NTR embryos. Whole-mount immunostaining for kdrl:GFP (first column) to show endocardium, MF-20 (second column) to show myocardium, and activated cleaved Caspase-3 (third column) to show apoptotic cells, at 36 hpf. Embryos were treated under the same conditions, in the presence of 1% DMSO (A) or of metronidazole (B), to induce cell specific apoptosis in myl7:nitroreductase expressing myocardial cells. Note that MTZ treated embryos had caspase-positive cardiac cells that are specifically in the myocardial (B, arrowheads, right column) and not the endocardial layer. |
|
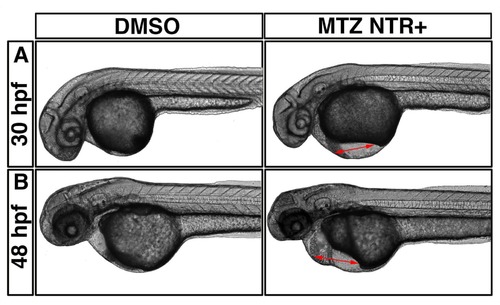
MTZ treated myl7:mCherry-NTR expressing embryos have defective cardiac function. Live imaging of DMSO treated (left column) and MTZ treated (right column) embryos at 30 hpf (A) and 48 hpf (B). Note the pericardial edema (right panels, red arrows). Lateral view, anterior is to the left. |
|
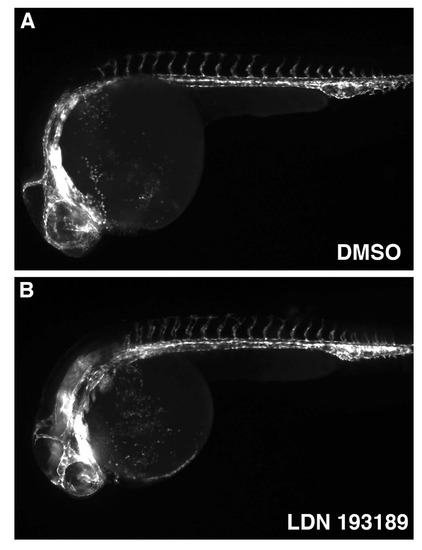
General vascular patterning is not significantly affected in LDN193189-treated embryos as observed in live fli1:GFP embryos at 24 hpf. Treatments were started at the tailbud stage (10 hpf). |