- Title
-
Zebrafish cerebrospinal fluid mediates cell survival through a retinoid signaling pathway
- Authors
- Chang, J.T., Lehtinen, M.K., Sive, H.
- Source
- Full text @ Dev. Neurobiol.
|
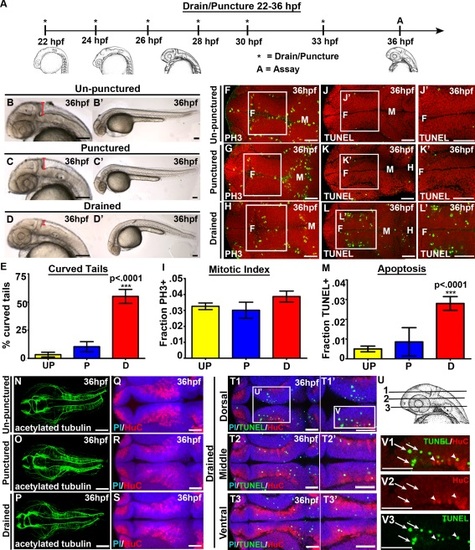
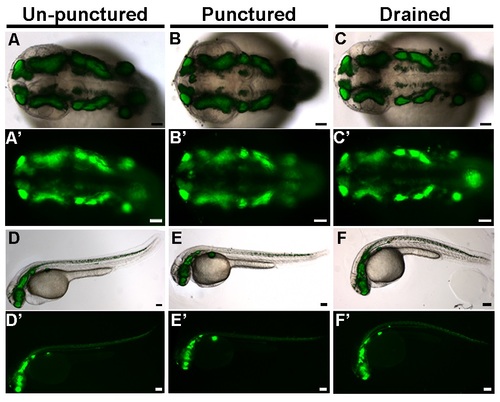
CSF promotes cell survival and tail extension. (A) Experimental design. Drainage/puncture (*) occurred every 2 hours from 22 to 36 hpf. Embryos were assayed (A) at 36 hpf. (B–D) Brightfield lateral view of unpunctured (B), punctured (C), and drained (D) embryos. (B′–D′) Whole embryo phenotype. (E) Percent of embryos with curved tails. (F–H) Dorsal view of PH3 (green) and (J–L) TUNEL (green). Propidium iodide in red. (F-H; J-L) white box indicates region quantified. (J′,K′L′) Magnified image of region indicated by white box in J,K,L. (I,M) Quantification of the fraction of PH3 (I) and TUNEL (M) positive cells. (N–P) Dorsal view of acetylated tubulin (green) or (Q–S) HuC (red) and propidium iodide (blue). (T) Dorsal/ventral sections of neural tube (T1-3) stained with TUNEL (green), HuC (red) and propidium iodide (blue). (U) Representative dorsal/ventral sections of neural tube shown in T. 1-3 correspond to T1-T3. (V) Magnified image of TUNEL and HuC (V1), HuC alone (V2) and TUNEL alone (V3) from dorsal section of diencephalon (T1). (N-S) projection overlaid on single slice of propidium iodide image, all other images are a single 0.6 µm slice. Data represented as mean ± SEM. F= forebrain, M = midbrain. UP = unpunctured, P = punctured, D = drained. Scale bars = 50 µm. |
|
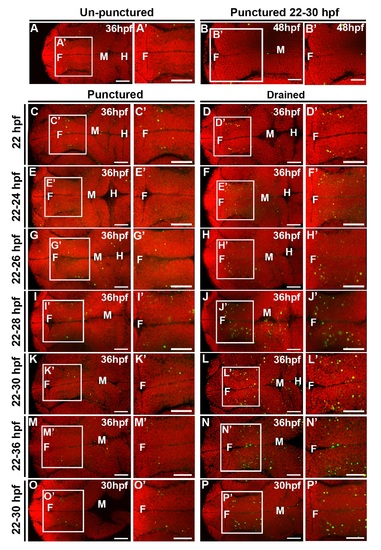
CSF is required from 25 to 30 hpf, while cell death persists at 48 hpf. (A) Quantification of TUNEL. X axis indicates puncture/drainage period. All assayed at 36 hpf. (B) Quantification of TUNEL at 30 hpf after puncture/drainage from 22 to 30 hpf. (C–E) Brightfield dorsal and lateral (C′–E′) images of 48 hpf embryos after puncture/drainage from 22 to 36 hpf. (F) Quantification of TUNEL at 48 hpf. (G–J) Dorsal view of TUNEL (green) and propidium iodide (red) in 48 hpf embryos after puncture/drainage from either 22–36 hpf (H–I) or 22–30 hpf (J) or unpunctured (G). Data represented as mean ± SEM. F = forebrain, M = midbrain. UP = unpunctured, P = punctured, D = drained. Scale bars = 50 µm. |
|
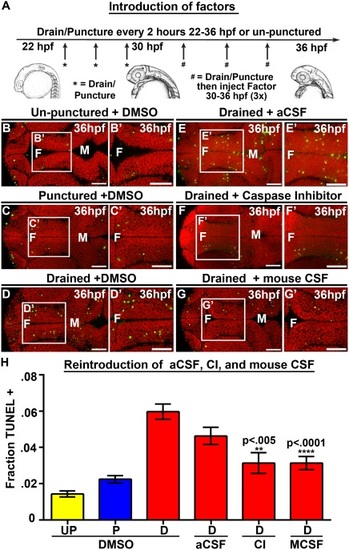
Introduction of Caspase 3 inhibitor and mouse CSF but not aCSF prevents cell death. (A) Experimental design. Drainage/puncture occurred every 2 hours from 22 to 36 hpf. From 30 to 36 hpf a factor was injected every 2 hours into the brain ventricles after drainage/puncture. Embryos were assayed at 36 hpf. (B–G) Dorsal view of TUNEL (green) and propidium iodide (red) in un-punctured (B) and punctured (C) embryos injected with DMSO or drained embryos injected with DMSO (D) aCSF (E), Caspase 3 inhibitor (F), or E10.5 mouse CSF (G). (H) Quantification of TUNEL. Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained, CI = Caspase 3 inhibitor, MCSF = E10.5 mouse CSF. Scale bars = 50 µm. |
|
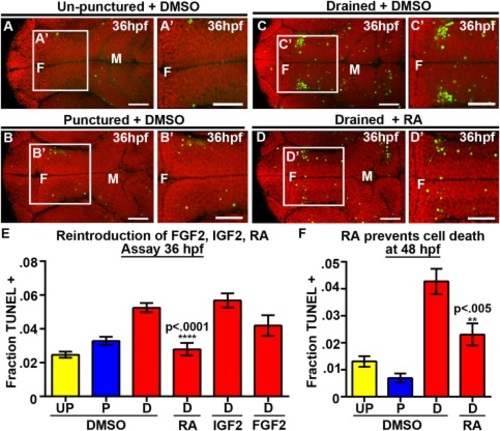
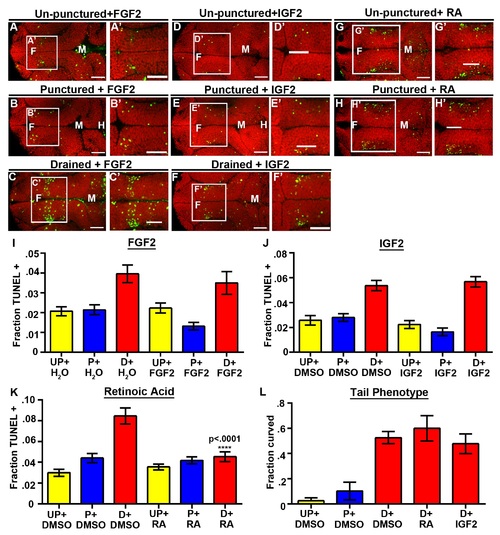
RA prevents neuroepithelial cell death at 36 and 48 hpf. (A–D) Dorsal view of TUNEL (green) and propidum iodide (red) of un-punctured (A), punctured (B) or drained embryos (C) injected with DMSO and drained embryos injected with RA (D). (E–F) Quantification of cell death at 36 hpf after introduction of FGF2, IGF2, or RA (E) or at 48 hpf after introduction of RA (F). Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained. Scale bars = 50 µm. |
|
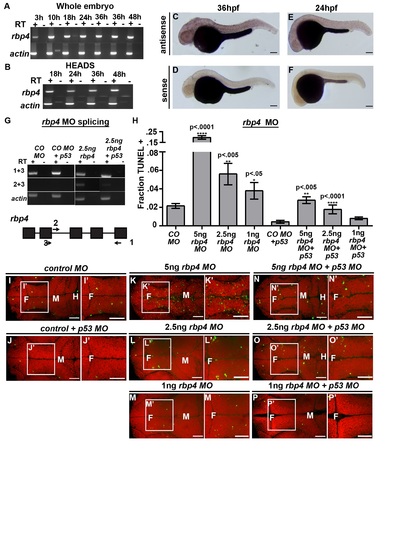
Loss of Rbp4 increases cell death. (A) Rbp4 sequence coverage from mass spectrometry (red). (B–E) Brightfield dorsal and lateral (B2–E2) view of control MO (B), control MO + p53 MO (C), rbp4 MO (D) and rbp4 MO + p53 MO (E) embryos. (F) Quantification of TUNEL after rbp4 MO and mRNA rescue. (G–J, L–O, R–S) Dorsal view of TUNEL (green) and propidium iodide (red). (G–J) TUNEL staining in control MO + p53 MO (G), control MO + rbp4 mRNA+ p53 MO (H), rbp4 MO+ p53 MO (I), rbp4 MO + rbp4 mRNA + p53 MO (J). (K) Method for RA rescue of rbp4 MO. (L–O) TUNEL staining in control MO + p53 MO + DMSO (L) control MO + p53 MO + RA (M), rbp4 MO+ p53 MO + DMSO (N), rbp4 MO + p53 MO + RA (O). (P) Quantification of TUNEL in RA rescue of rbp4 MO. (Q) Method of injection of Rbp4 inhibitor, A1120. (R-S) TUNEL staining in DMSO (R) or A1120 (S) injected embryos (T) Quantification of TUNEL in A1120 or DMSO injected embryos. Data represented as mean ± SEM. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 µm. |
|
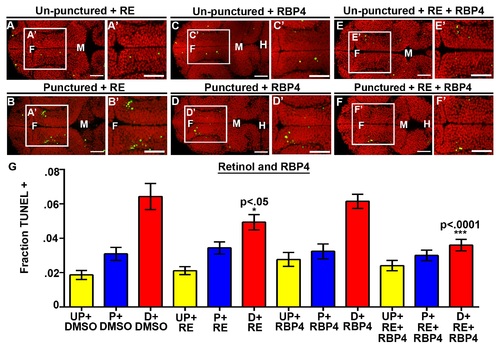
Rbp4 is required for cell survival. (A–F) Dorsal view of TUNEL (green) and propidium iodide (red) in unpunctured (A), punctured (B) and drained (C) embryos injected with DMSO or drained embryos injected with RBP4 only (D), retinol (RE) only (E), or combination of retinol (RE) and Rbp4 (F). (G) Quantification of TUNEL after RBP4/retinol injection. Data represented as mean ± SEM. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 µm. |
|
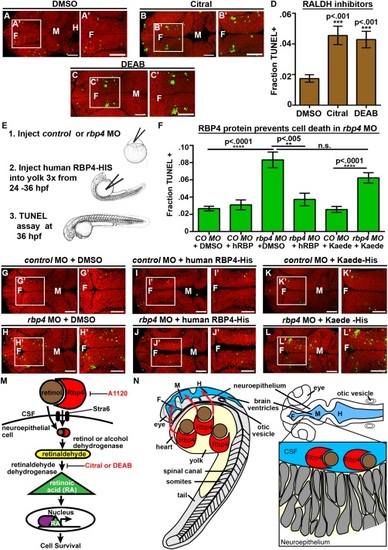
RA synthesis is required for cell survival and Rbp4 can be transported from the yolk to the CSF. (A–C) Dorsal view of TUNEL (green) and propidium iodide (red) in embryos injected with DMSO (A), Citral (B) or DEAB (C). (D) Quantification of TUNEL after injection of inhibitors into wild-type embryos every two hours from 22 to 36 hpf. (E) Experimental design for injection of human RBP4 protein into yolk of rbp4 morphants. (F) Quantification of TUNEL positive cells in rbp4 morphants after injection of RBP4 or Kaede protein. (G–L) Dorsal view of TUNEL (green) and propidium iodide (red) in control MO + p53 MO (G,I,K) or rbp4 MO+ p53 MO (H,J,L) embryos injected with DMSO (G,H), human RBP4-His protein (I,J) or Kaede-His protein (K,L). (M) Model of retinoid signaling pathway in the embryonic brain. Inhibitors used in red. (N) Model of Rbp4 + RA precursor transport. Rbp4 bound to retinol/RA precursors can be transported from the yolk to CSF (left, red arrows) where it is taken up from the CSF by a RA metabolizing cell, oxidized into RA and promotes cell survival (right). Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained. Scale bars = 50 µm. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|