- Title
-
Zebrafish models for nemaline myopathy reveal a spectrum of nemaline bodies contributing to reduced muscle function
- Authors
- Sztal, T.E., Zhao, M., Williams, C., Oorschot, V., Parslow, A.C., Giousoh, A., Yuen, M., Hall, T.E., Costin, A., Ramm, G., Bird, P.I., Busch-Nentwich, E.M., Stemple, D.L., Currie, P.D., Cooper, S.T., Laing, N.G., Nowak, K.J., Bryson-Richardson, R.J.
- Source
- Full text @ Acta Neuropathol.
|
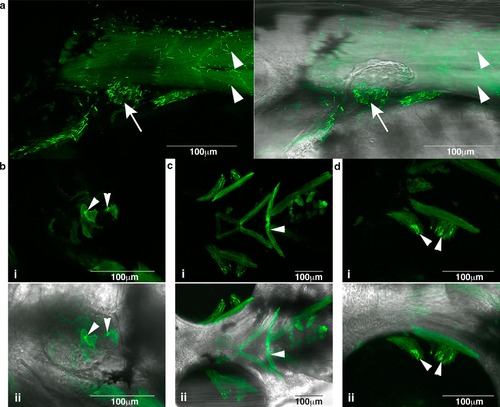
Nemaline bodies form in skeletal muscle in Tg(ACTA1 D286G-eGFP)high zebrafish. Nemaline bodies were detected in a skeletal muscle (arrowheads) and pectoral fins (arrow) (i and ii overlaid with brightfield), b heart (arrowheads) (i and ii overlaid with brightfield), c facial muscles (arrowheads) (i and ii overlaid with brightfield) and d ocular muscles (arrowheads; i and overlaid with ii brightfield) EXPRESSION / LABELING:
PHENOTYPE:
|
|
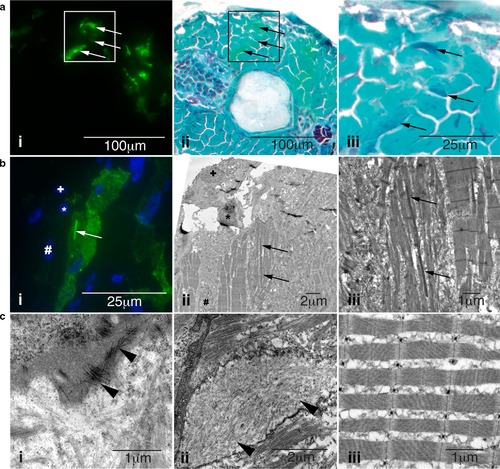
Characterization of skeletal muscle pathology in Tg(ACTA1D286G-eGFP)high zebrafish. a Skeletal muscle expressing i mosaic ACTA1D286G-eGFP, and ii overlaid with a light microscopy image of the same section showing Gomori trichrome staining, and iii enlarged. Dark regions (indicative of nemaline bodies) of disrupted muscle correspond to eGFP expression (arrows). b Correlative light and electron microscopy of Tg(ACTA1D286G-eGFP)high fish muscle at 2 dpf. b i Fluorescent image and corresponding ii electron microscopy image of skeletal muscle section containing a dense, elongated nemaline body (arrow) and enlarged in (iii). Sections are matched using nuclei positions (asterisk, plus and hash). c i Accumulations of actin filaments (arrowheads) and ii diffuse regions of filamentous actin (arrowheads), as well as ii disrupted sarcomeric regions are evident in Tg(ACTA1 D286G-eGFP)high skeletal muscle, at 2 dpf unlike the iii uniform sarcomeres observed in Tg(ACTA1-eGFP) zebrafish |
|
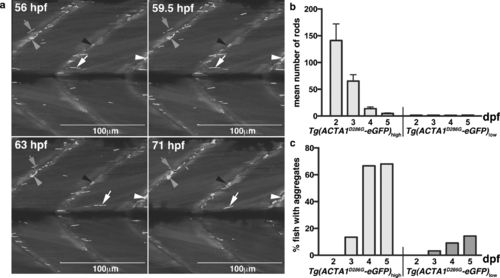
Formation of nemaline bodies and aggregates in Tg(ACTA1 D286G-eGFP)high zebrafish. a Maximum projection images from time lapse of Tg(ACTA1D286G-eGFP)high fish from 56 to 71 hpf showing nemaline bodies distributed throughout the skeletal muscle (arrows). Nemaline bodies’ fragment from 59.5 hpf (arrows), coincident with the formation of aggregates at the myosepta (arrowheads). b Quantification of the mean number of nemaline bodies in Tg(ACTA1 D286G-eGFP) low (n = 50 per stage) and Tg(ACTA1 D286G-eGFP) (n = 48 per stage) strains. c Quantification of the percentage of fish displaying globular aggregates in Tg(ACTA1 D286G-eGFP) low and Tg(ACTA1 D286G-eGFP) high strains (n = 50 per stage). Error bars represent SEM from three independent experiments (n = 45 per replicate) |
|
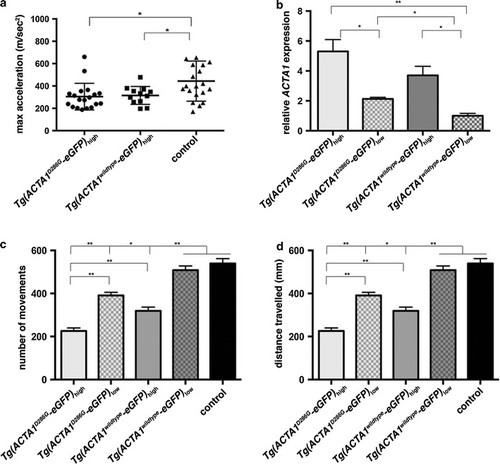
Quantification of muscle function in Tg(ACTA1-eGFP) zebrafish. a Quantification of the maximum acceleration recorded from touch-evoked response assays of Tg(ACTA1D286G-eGFP)high and Tg(ACTA1wildtype-eGFP)high zebrafish compared to control zebrafish at 2 dpf. Error bars represent SD for n = 15–19 zebrafish, *p < 0.05. b qRT-PCR analysis of ACTA1-eGFP expression in transgenic lines at 2 dpf. No significant difference was observed between Tg(ACTA1D286G-eGFP)high and Tg(ACTA1wildtype-eGFP)high zebrafish. Error bars represent ±SEM for four replicate experiments with each experiment comprising a pooled samples of 20 fish, *p < 0.05, **p < 0.01. c, d Quantification of the c number of small movements and d distance traveled by Tg(ACTA1D286G-eGFP)high and Tg(ACTA1wildtype-eGFP)high and Tg(ACTA1D286G-eGFP)low and Tg(ACTA1wildtype-eGFP)low strains compared to control fish at 6 dpf. Error bars represent ±SEM for three replicate experiments (n = 48 per experiment), *p < 0.05, **p < 0.01 |
|
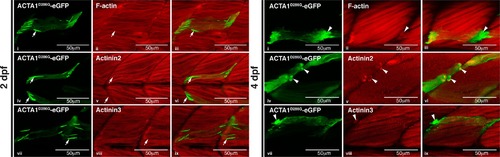
Characterization of nemaline bodies and aggregates in ACTA1-eGFPD286G muscle in zebrafish. At 2 dpf, mosaic expression of ACTA1D286G-eGFP in the muscle (green) results in the formation of nemaline bodies (arrows; i, iv, vii) that do not stain with an actinin2 antibody (red; ii and overlaid in iii), actinin3 antibody (red; v and overlaid in vi), or phalloidin (labeling F-actin, red; viii and overlaid in ix) despite correct localization of these markers in the sarcomere. At 4 dpf, mosaic expression of ACTA1D286G-eGFP results in the formation of globular aggregates (arrowheads; i, iv, vii) in the muscle (green) stain with an actinin2 antibody (red; ii and overlaid in iii), actinin3 antibody (red; v and overlaid in vi) and phalloidin (red; viii and overlaid in ix) |
|
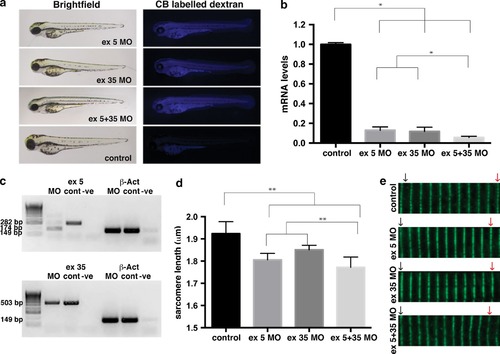
Analysis of nebulin knockdown in zebrafish muscle. a Brightfield and fluorescent images of wild-type embryos injected with two different nebulin (neb)-targeting morpholinos (MOs) compared to control uninjected embryos at 2 dpf. Successful injection is confirmed by the presence of Cascade Blue (CB) labeling. b qRT-PCR analysis showing significant knockdown of neb transcript in Neb morphants compared to control uninjected (control) at 2 dpf. Error bars represent ±SEM for three replicate experiments with each experiment comprising a pooled samples of 20 fish, *p < 0.05. c RT-PCR analysis revealed the absence of correctly spliced, and presence of mis-spliced, neb transcript in Neb ex5 morphants compared to control uninjected (cont) and reduced expression of neb transcript in Neb ex 35 morphants at 2 dpf. β-actin was used as an amplification control. d Quantification of sarcomere lengths showed a significant decrease in neb morphants compared to controls at 2 dpf. Error bars represent ± SEM for four replicate experiments (n = 10 per experiment), *p < 0.01. e Actinin2 labeling of Z-disks in morphant and control zebrafish at 2 dpf illustrates the reduced sarcomere length in Nebulin morphants compared to controls over 10 sarcomeres (represented by the distance between the black arrow and red arrows) |
|
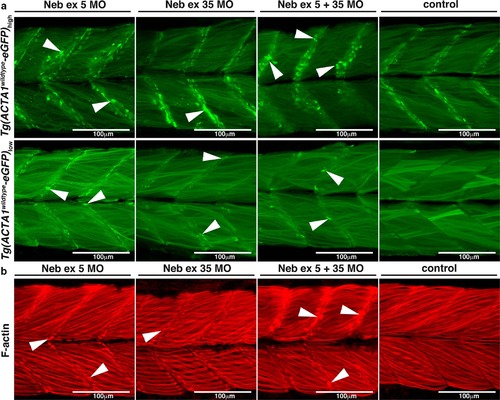
a Maximum projection confocal microscopy images of Tg(ACTA1wildtype-eGFP)low and Tg(ACTA1wildtype-eGFP)high zebrafish strains injected with two different Nebulin (Neb) morpholinos compared to control uninjected embryos at 2 dpf. There is an increased prevalence of eGFP-positive globular aggregates at the myosepta (arrowheads) in Tg(ACTA1wildtype-eGFP)high Neb morphants compared to controls. Knockdown of Neb produces globular aggregates at the myosepta (arrowheads) in Tg(ACTA1wildtype -eGFP)low Neb morphants that are absent in control uninjected embryos. b Maximum projection confocal microscopy images of wild-type embryos injected with Neb morpholinos at 2 dpf and stained with phalloidin shows an increase in actin-positive aggregates at the myosepta (arrowheads), which are absent in control uninjected embryos |
|
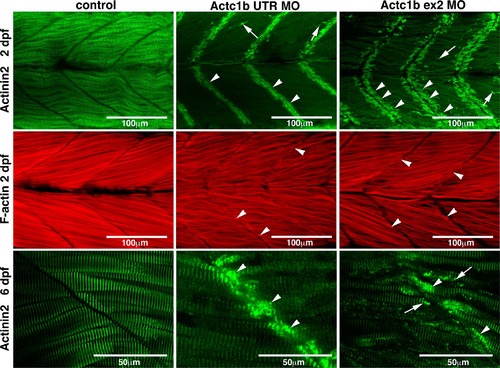
Maximum projection confocal microscopy images of Actc1b morphants and control zebrafish stained with phalloidin at 2 dpf or with an actinin2 antibody at 2 dpf and 6 dpf. Actinin2 and phalloidin-positive nemaline bodies are observed throughout the muscle fibers (arrows) as well as projecting from the myosepta (arrowheads) in Actc1b morphants compared to controls |
|
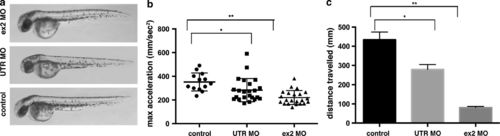
Functional analysis of Actc1b knockdown in zebrafish. a Brightfield images of wild-type embryos injected with actc1b targeting morpholinos (MO) compared to control uninjected embryos. b Quantification of the maximum acceleration recorded from touch-evoked response assays of Actc1b morphants compared to control zebrafish at 2 dpf. Error bars represent SEM for three replicate experiments (20 fish per replicate), **p < 0.01. c Quantification of the distance traveled by Actc1b morphants compared to control zebrafish at 6 dpf. Error bars represent ±SEM for three replicate experiments (n = 48 per experiment), **p < 0.01 |
|
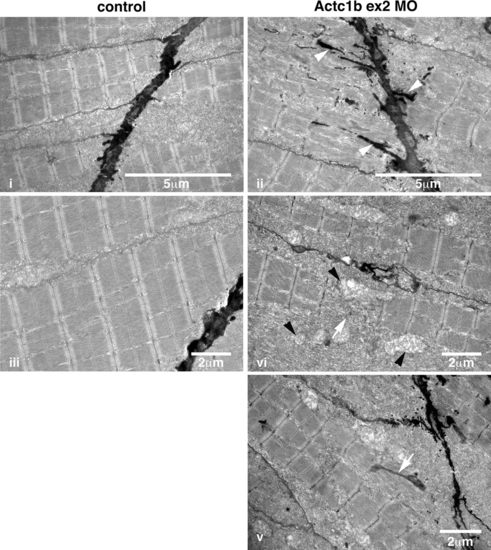
Electron microscopy images of Actc1b ex 2 morphants at 2 dpf showing ii electron-dense projections emanating from the myosepta (arrowheads) as well as nemaline bodies derived from v thickened Z-disks throughout the fibers not observed in control fish (i, iii). Sections also contained broken muscle fibers iv as well as numerous mitochondria (black arrowheads; iv), compared to the uniform sarcomeres in control zebrafish skeletal muscle iii PHENOTYPE:
|
|
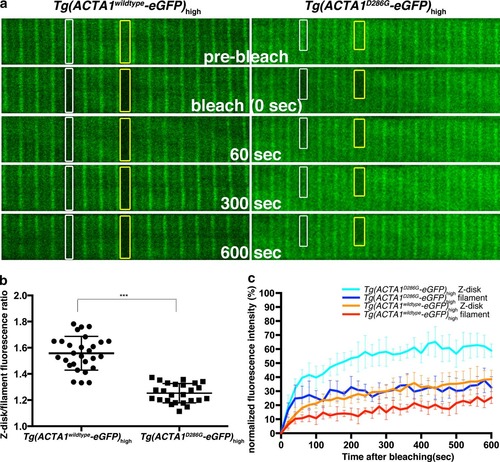
Fluorescence recovery after photobleaching (FRAP) analyses of ACTA1 and ACTA1D286G. a Confocal images of ACTA1-eGFP localization at the Z-disk (white boxes) and along the thin filament (yellow boxes) in single muscle fibers of Tg(ACTA1 D286G-eGFP)high and Tg(ACTA1 wildtype -eGFP) high embryos at 2 dpf. Image sequence shows ACTA1-eGFP prior to photobleaching (pre-bleach), at the time of photobleaching (bleach, 0 s), and 60, 300 and 600 s following photobleaching. Prior to photobleaching eGFP in Tg(ACTA1 wildtype-eGFP) high muscle is primarily localized to the Z-disk (white boxes), whereas in Tg(ACTA1 D286G-eGFP) high fibers, eGFP expression is more diffuse throughout the filament (yellow boxes). b Quantification of the fluorescence intensity at the Z-disk compared to the filament in Tg(ACTA1 D286G -eGFP) high and Tg(ACTA1 wildtype -eGFP) high muscle fibers. Error bars represent SD for 12 animals (quantifying 2 fibers per animal), ***p < 0.001. c Recovery profiles for ACTA1-eGFP and ACTA1D286G-eGFP at the Z-disk and filament. Error bars represent SD for 8–10 animals (quantifying 2 fibers per animal) |
|
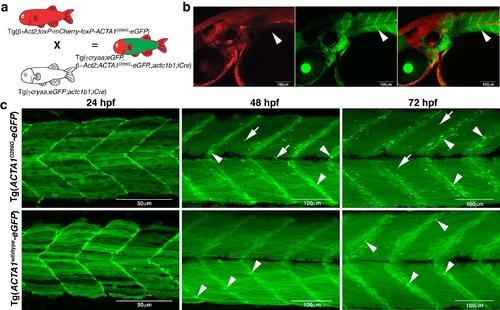
Generation and phenotypic characterization of Tg(ACTA1-eGFP) stable lines. a) Crossing scheme to generate Tg(ACTA1-eGFP) fish. b) Confocal images show that upon iCre-mediated deletion of the loxP cassette, containing mCherry, eGFP is localized to the muscle (green) and does not overlap with other tissues (red; arrowheads). c) Maximum projection confocal microscopy images showing expression of ACTA1-eGFP in zebrafish skeletal muscle at 24, 48, and 72 hpf. Tg(ACTA1D286G-eGFP)high expression results in the formation of nemaline bodies (arrows) at 48 hpf and aggregates at the myosepta (arrowheads). No nemaline bodies are observed in Tg(ACTA1wildtypeeGFP)high skeletal muscle at any stage however globular aggregates are evident from 48 hpf at the myosepta (arrowheads). |
|
Characterization of nemaline bodies and aggregates in Tg(ACTA1-eGFP)high zebrafish. a) Confocal microscopy images of nemaline bodies and aggregates in Tg(ACTA1D286G-eGFP)high muscle. At 2 dpf, nemaline bodies (arrows) in (i) Tg(ACTA1D286G-eGFP)high muscle (green) do not stain with Actinin2 (red; ii and overlaid in iii) or phalloidin (labeling F-actin) (red; i and overlaid in ii) despite correct localization of these markers in the sarcomere. At 4 dpf, aggregates (arrowheads) in (i) Tg(ACTA1D286G-eGFP)high muscle (green) stain for Actinin2 (red; ii and overlaid in iii), and phalloidin (red; ii and overlaid in iii) in Tg(ACTA1D286G-eGFP)high muscle (green). Co-injection of actc1b;ACTA1D286G-eGFP and actc1b;actc1a-mCherry (cardiac α-actin) results in mosaic expression of both constructs throughout the skeletal muscle. ACTA1D286G-eGFP (green) and cardiac actin (red) co-localize in nemaline bodies at 2 dpf (arrows; ii and overlaid in iii) and in aggregates at 4 dpf in (arrowheads; ii and overlaid in iii). b) Confocal microscopy images of globular aggregates in Tg(ACTA1wildtype-eGFP)high muscle. At 2 dpf (arrows), and 4 dpf, aggregates (arrowheads) are observed in (i) Tg(ACTA1wildtype-eGFP)high skeletal muscle (green) labeled with Actinin2 (red; ii and overlaid in iii) and phalloidin (red; ii and overlaid in iii). |