- Title
-
Zebrafish foxc1a drives appendage-specific neural circuit development
- Authors
- Banerjee, S., Hayer, K., Hogenesch, J.B., Granato, M.
- Source
- Full text @ Development
|
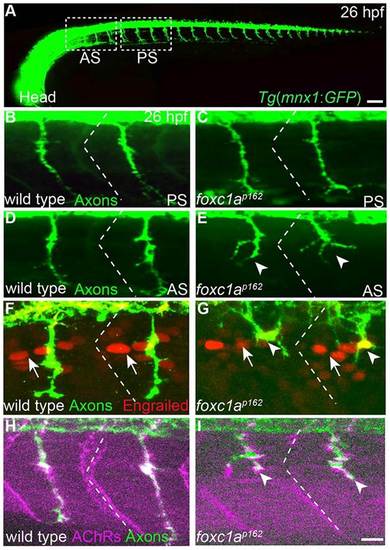
foxc1a guides primary motor axon selectively in anterior somite segments. (A) Lateral (composite) view of a 26-h-old Tg (mnx1:GFP) embryo expressing GFP in all motor neurons. White dashed boxes mark anterior somite segments (AS) and posterior segments (PS) considered in this study. Compared with wild type (B,D), foxc1a mutants (C,E) exhibit motor axon guidance defects selectively in anterior but not posterior somitic segments. (F-I) Somite polarity (F,G), as revealed by the localization of Engrailed-positive nuclei (arrows) towards the anterior somite boundary (dashed lines), and muscle differentiation (H,I), as revealed by the apposition of muscle AChRs with axons to form en passant synapses are unaffected in foxc1a mutants. Arrowheads indicate stalled and branched axons. Scale bars: 50µm in A; 10µm in B-I. |
|
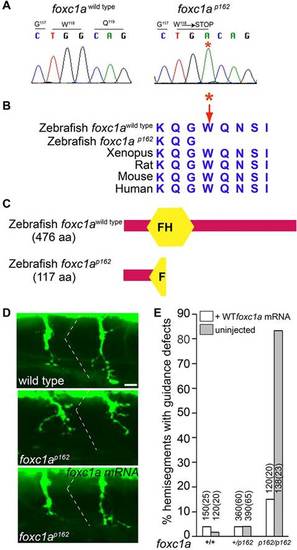
The p162 mutant phenotype is caused by a nonsense mutation in the foxc1a gene. (A,B) The G to A nucleotide mutation converts tryptophan at position 118 into a premature stop codon. (C) Conceptual translation of the foxc1ap162 predicts a 117 amino acid protein truncated within the forkhead (FH) domain. (D) Injection of wild-type foxc1a mRNA restores axon guidance in foxc1a mutants. (E) Quantification of the mRNA rescue. Numbers on top of individual bars indicate the number of hemisegments and embryos (brackets) analyzed. For all genotypes, only the first six anterior segments were analyzed. Scale bar: 10µm in D. |
|
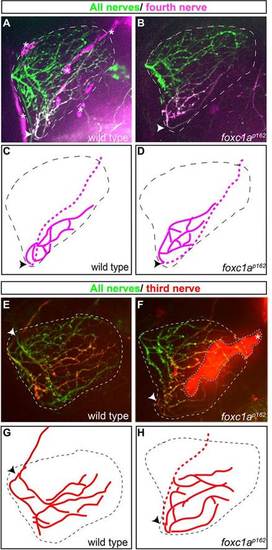
foxc1a is required for fin-innervating motor nerves to convert at the plexus. (A) Lateral (composite) view of a 96-hpf-old Tg(mnx1:GFP) larvae expressing GFP in all motor nerves. Yellow dashed boxes mark anterior segments (AS) and posterior segments (PS) considered in this study; white dotted area outlines the pectoral fin. (B,C) Compared with wild-type larvae, trunk innervation (posterior segments) is unaffected in foxc1a mutants. (D-G) By contrast, three out of the four fin-innervating nerves in foxc1a mutants display axonal defects at 48hpf (D-G) and at 96hpf (H-K). Dotted areas outline the pectoral fin, arrowheads indicate the position of the fin plexus and arrows indicate the fourth pectoral fin nerve. Cyan asterisks indicate ectopic fasciculation; red asterisks mark defasciculation. Scale bars: 50µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
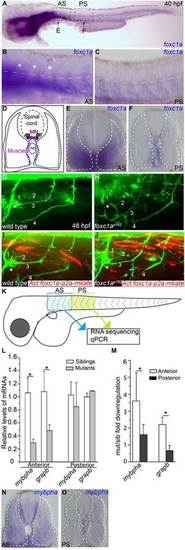
foxc1a is expressed and functions in somitic muscle to guide fin nerves. (A-C) At 40hpf, foxc1a mRNA is highly enriched in anterior (A,B) but not posterior somite segments (A,C). (B,C) Magnified views of anterior and posterior somitic segments shown in A, asterisks indicate the anterior most three segments. (D-F) Cross-sections through anterior and posteriors segments (at the level of arrows in A) reveal foxc1a mRNA accumulation in muscle fibers located along the fin nerve path. (G-J) Stochastic expression of a foxc1a-p2a-mKate transgene in muscle fibers (I,J) restores fin nerve guidance. (K-O) RNA-seq identifies transcripts selectively enriched in anterior segments and downregulated in foxc1a mutants, as confirmed by real-time PCR experiments (L,M; asterisks indicate significance; P<0.05, Student′s t-test) and by in situ hybridization of mybpha (N, cross-section through anterior segment; O, cross-section through posterior segment). Data are mean±s.e.m. |
|
Motor axons select their synaptic target independently of their migration history. (A-D) In wild type and foxc1a mutants, photoconverted Kik-GR-positive axons of the fourth fin nerve enter the fin at the ventral point of the fin and project to their ventroposterior target area. Arrowheads indicate where the nerve enters the fin. (E-H) In foxc1a mutants, photoconverted Kik-GR-positive axons of the third fin nerve enter the fin at an ectopic, ventral point of the fin yet project to the same target area as the corresponding wild-type axons that entered through the endogenous dorsal fin entry point. Red and magenta lines show tracing of photoconverted third and fourth nerves, respectively. Dotted lengths of red and magenta lines denote regions of the nerve underneath the fin. Asterisks (in A and F) and cyan dashed area (F) mark non-neural tissues giving background fluorescence in red channel. Scale bar: 50µm. |
|
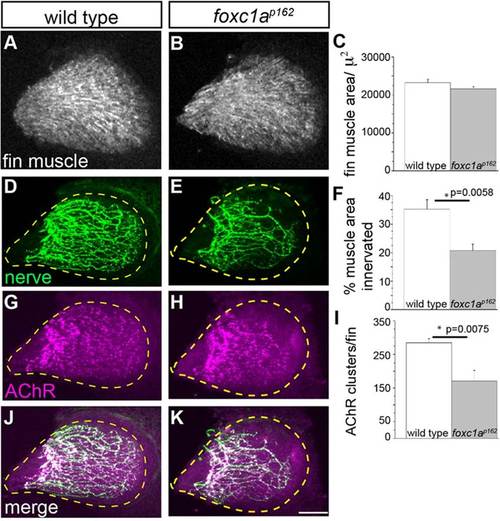
foxc1a mutants display reduced fin nerve arborization and synaptic contacts. (A-C) At 96hpf, fin muscle differentiation and surface area are unaffected in foxc1a mutants compared with wild type. (D-K) By contrast, the area of the fin muscle covered by presynaptic nerve branches (D-F) and the density of postsynaptic AChR clusters is significantly reduced in foxc1a mutants. All images are maximum projection views. The fin is outlined using a dashed yellow line. Scale bar: 50µm. (C,F,I) Asterisks indicate P<0.05 (Student′s t-test). Data are mean±s.e.m. |
|
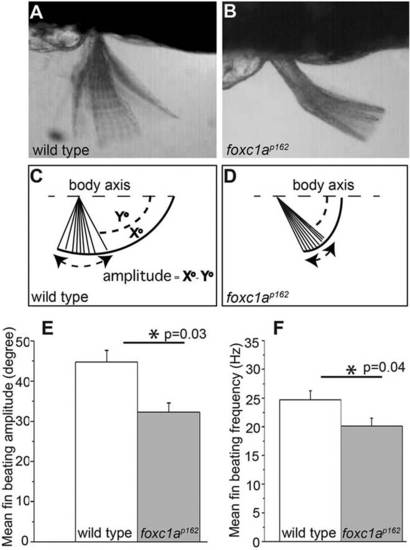
foxc1a mutants exhibit reduced fin mobility. (A,B) Time projections from high-speed video recordings of 96hpf wild type and foxc1a mutants reveal reduced pectoral fin mobility. (C,D) To quantify the defects, we defined the fin-beating amplitude as X°-Y°, where X° designates the maximum elevation angle of fin with respect to body axis (dashed line) in a given beating cycle, and Y° denotes the maximum depression angle in a given beating cycle. (E,F) foxc1a mutants display a significant reduction of both beating amplitude and frequency of pectoral fins. (C,F,I) Asterisks indicate P<0.05 (Student′s t-test). PHENOTYPE:
|