- Title
-
The dlx5a/dlx6a Genes Play Essential Roles in the Early Development of Zebrafish Median Fin and Pectoral Structures
- Authors
- Heude, E., Shaikho, S., Ekker, M.
- Source
- Full text @ PLoS One
|
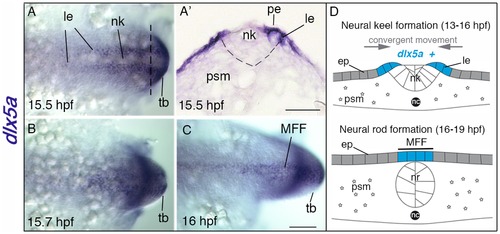
Expression of dlx5a during early specification of median fin fold ectodermal cells. (A–C) In situ hybridization for dlx5a in zebrafish embryos from 15.5 hpf to 16 hpf: dorsal view of the posterior axis (A, C) and coronal section (A2) at the level indicated by the dashed line in (A). At 15.5 hpf, dlx5a is expressed in ectodermal cells at the lateral edges (le) of the neural keel (nk) underlying the periderm (pe) (A, A2) (the dashed line in A2 delineates the neural keel). From 15.5 hpf to 16 hpf, ectodermal cells expressing dlx5a follow a dynamic convergent movement to form the presumptive median fin fold (MFF) at the dorsal midline of the embryo (B–C). (D) Schematic representation of the zebrafish dorsal cellular movement implicating dlx5a based on A–C. The convergent movement produced by the establishment of the neural rod (nr) (16–19 hpf) leads to the fusion of the two lateral edges at the midline into the presumptive MFF expressing dlx5a. ep, epidermis; nc, notochord; psm, presomitic mesoderm; tb, tail bud. Scale bars shown in C for A–C and in A2 50 μm. EXPRESSION / LABELING:
|
|
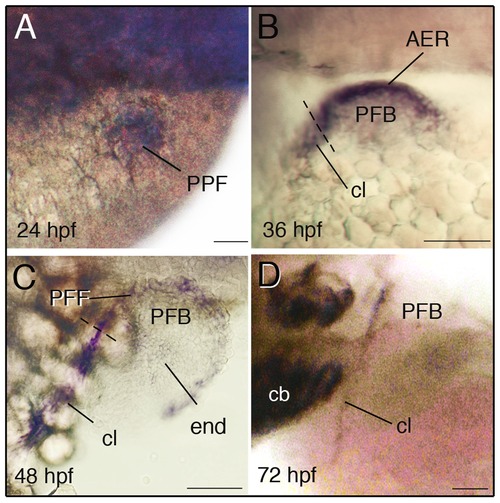
Expression of dlx5a during zebrafish pectoral development. Whole mount in situ hybridization for dlx5a in the pectoral region of zebrafish embryos from 24 hpf to 72 hpf. During pectoral fin formation, dlx5a is expressed in apical ectodermal cells of the presumptive pectoral fin bud (PPF) at 24 hpf (A) and in the apical ectodermal ridge (AER) of the early pectoral fin bud (PFB) at 36 hpf (B). At 48 hpf, dlx5a expression is detected in the pectoral fin fold (PFF) and weak expression is observed in endochondral cells (end) of the PFB. Moreover, the transcripts are detected in the developing cleithrum (cl) from 36 hpf to 72 hpf (B–D). The dashed lines in B–C indicate the limit between the AER/PFF structures and cleithrum precursor cells. Note the absence of dlx5a expression in the PFF at 72 hpf (D). Scale bars 50 μm. EXPRESSION / LABELING:
|
|
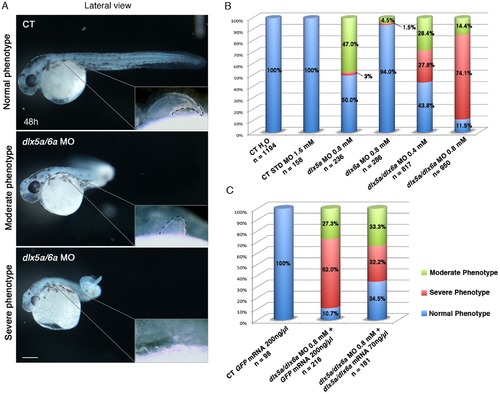
Phenotypes obtained with different dlx5a/dlx6a morpholinos and mRNA treatments. (A) Phenotypes observed in dlx5a/6a morphant embryos. Lateral view of control (CT) and dlx5a/6a morphant embryos at 48 hpf. The dlx5a/6a gene knock down results in moderate “curved tail” and severe “ curly tail” phenotypes compared to controls. The moderate and severe phenotypes are associated with hypoplasia and agenesis of pectoral fin bud respectively as shown in the pectoral region magnifications. Scale bar for all panels 100 μm. (B, C) The graphics show the percentages of normal (blue bars), moderate (green bars) and severe (red bars) phenotypes obtained at 48 hpf following injection of different dlx5a/dlx6a MOs and dlx5a/dlx6a mRNAs. For each treatment, the number (n) of specimens analyzed is indicated and each experiment was performed at least 3 times. The B graph shows the following treatments: control embryos injected with H2O; control embryos injected with a control MO (1.6 mM); single morphants injected with either dlx5a or dlx6a MOs (0.8 mM); double morphants co-injected with dlx5a and dlx6a MOs at two different concentrations (0.4 mM or 0.8 mM each). The C graph shows rescue experiments: control embryos injected with GFP mRNA (200 ng/ μl); control embryos co-injected with dlx5a/6a morpholinos (0.8 mM each) and GFP mRNA (200 ng/ µl) and embryos co-injected with dlx5a/6a morpholinos (0.8 mM each) and dlx5a/dlx6a mRNAs (70 ng/ μl each). PHENOTYPE:
|
|
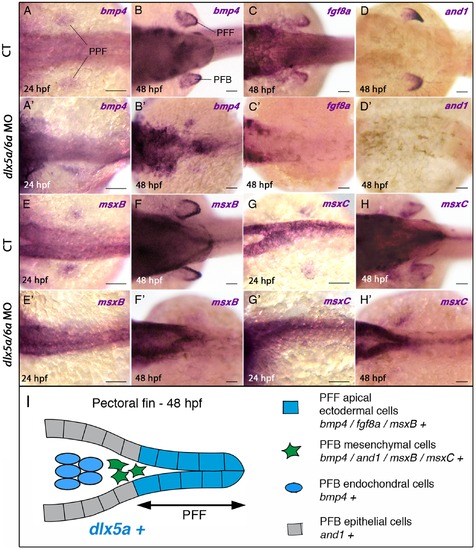
Impaired expression of bmp4, fgf8a, and1 and msx genes in the pectoral fin region of dlx5a/6a morphants. Whole mount in situ hybridization for bmp4 (A, A2–B, B2), fg8a (C, C2), and1 (D, D2), msxB (E, E2–F, F2) and msxC (G, G2–H–H2) at 24 and 48 hpf in dorsal views of control (A–H) and dlx5a/6a morphant (A2–H2) embryos. At 24 hpf, bmp4, msxB and msxC genes are expressed in apical ectodermal cells of the presumptive pectoral fin bud (PPF) in control embryos (A, E, G). In dlx5a/6a morphants, bmp4 expression is lost or altered in the presumptive pectoral fin bud (A2) and the msxB and msxC transcripts are hardly detectable (E2, G2). In 48 hpf control embryos, bmp4 is expressed in the pectoral fin fold (PFF), the underlying mesenchyme and in mesodermal cells of the pectoral fin bud (PFB) (B). At the equivalent stage, fgf8a is detected in PFF ectodermal cells (C), and and1 expression is observed in the distal mesenchyme and in epithelial cells of the PFB but not in the PFF (D). The msxB gene is expressed in the PFF and the underlying mesenchyme (F), and msxC is detected in the mesenchymal cells but not in the PFF (H). In contrast to what is observed in controls at 48 hpf, dlx5a/6a morphants show a marked decrease or loss of expression of the PFB markers associated with pectoral fin agenesis (B2–D2, F2, H2). (I) Schematic representation of the pectoral fin bud at 48 hpf summarizing the expression of dlx5a and the analyzed PFB markers in their corresponding cellular types. Scale bars 50 μm. |
|
Knock down of dlx5a/6a leads to a defect of cleithrum differentiation. Dorsal views of whole mount in situ hybridization for runx2b and col10a1 on control (A, C, E) and dlx5a/6a morphant (B, D, F) embryos at 36 hpf (A–B) and 48 hpf (C–F). In controls at 36 hpf, runx2b is expressed in precursors cells of the cleithrum (cl) (A) whereas expression is absent in the pectoral region of dlx5a/6a morphants (B, black asterisks). At 48 hpf, expression of runx2b and col10a1 is detected in differentiating osteoblasts of the cleithrum which supports the pectoral fin bud (C, E). The runx2b transcripts are also observed at the craniofacial level in the opercular (op) and ceratobranchial 5 (cb5) bone precursors (C). In contrast, dlx5a/6a morphants show a drastic loss of runx2b and col10a1 expression in the pectoral region (D, F, black asterisks) and of runx2b expression at the craniofacial level (D, blue asterisks). Scale bar shown in F for all panels 100 μm. |
|
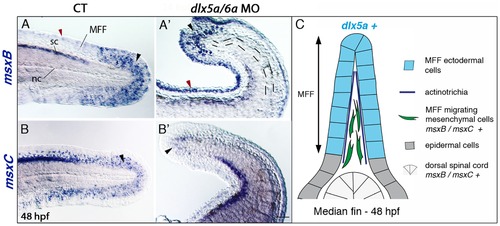
Impaired median fin fold expression of msx genes in dlx5a/6a morphants. Whole mount in situ hybridization for msxB (A, A2) and msxC (B, B2) in lateral views of the posterior axis of control (A–B) and dlx5a/6a morphant (A2–B2) embryos at 48 hpf. In controls, msxB and msxC genes are expressed in the spinal cord (sc) and in mesenchymal cells of the median fin fold (MFF) (black arrowheads A–B). Slight msxB expression is also observed in MFF apical cells (red arrowhead A). In morphants, msx expression is limited to a few distal MFF mesenchymal cells (black arrowheads A2–B2) and aberrant msxB expression is detected in the MFF apical cells (red arrowhead A2). The dashed lines in (A2) underlie the undulating and larger notochord (nc) displayed in the morphants. (C) Schematic representation of the dorsal median fin at 48 hpf summarizing the expression of dlx5a and msx genes in their corresponding cellular types. Scale bar shown in B2 for all panels 50 μm. |
|
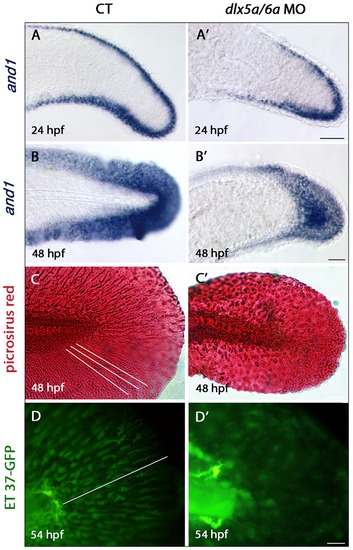
Defects in actinotrichia formation in the median fin fold of dlx5a/6a morphants. Lateral views of the developing median fin fold (MFF) of control (A–D) and dlx5a/6a morphant (A2–D2) embryos. Whole mount in situ hybridization for and1 at 24 (A, A2) and 48 hpf (B, B2). In control embryos, and1 is expressed in epithelial cells of the fin fold at 24 hpf and in all MFF cells at 48 hpf (A–B). In dlx5a/6a morphants, and1 expression is detected in the MFF but transcripts show a less extended antero-posterior expression domain at 24 hpf (A2). At 48 hpf, morphants display a thinner and1 expression domain in the ventral and dorsal part of the MFF (B2). The picrosirius red histological staining in 48 hpf control embryos reveals the MFF cellular organisation with the presence of actinotrichia (C, the white lines indicate their orientation and length). In the morphants, the MFF is smaller and granular associated with absence of actinotrichia (C2). The ET 37-GFP enhancer-trap line reveals the MFF mesenchymal cells displaying filopodia which migrate along the actinotrichia at 54 hpf (D, the white line indicates the direction of migration). The dlx5a/6a knock down in ET 37-GFP embryos leads to impaired MFF mesenchymal migration, the cells are disorganized and do not show filopodia. Scale bars in A–B 50 μm, C 20 μm, D 10 μm. |
|
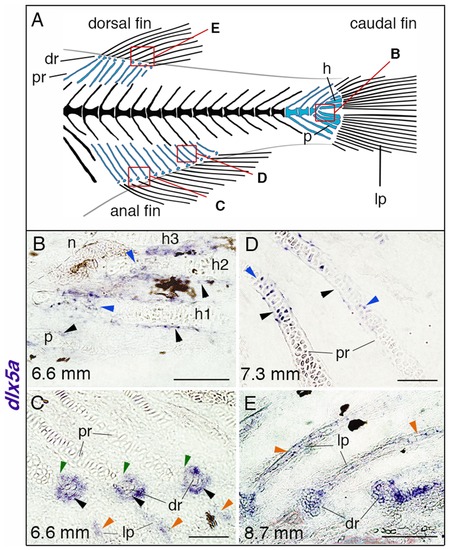
dlx5a expression during unpaired fin skeletogenesis. (A) Overview of the posterior axial skeleton of a one-month-old zebrafish. Endoskeletal fin supports are colored in blue and red squares indicate the structures analyzed in (B–E). (B–E) Whole mount in situ hybridization for dlx5a on 10 µm parasagittal frozen sections of late-stage zebrafish from 6.6 mm (16 days post-fertilization) to 8.7 mm (1 month post-fertilization). In 6.6 mm larvae, dlx5a is expressed in the perichondrium of the hypurals 1 to 3 (h1-3) and of the parahypural (p) (B, black arrowheads) as well as in maturing chondrocytes of h1 and h2 (B, blue arrowheads). The transcripts are also detected in cells surrounding the distal radials (dr) during radial segmentation (C, black arrowheads), notably in the zone of segmentation (C, green arrowheads), and in the developing lepidotrichia (lp, C, orange arrowheads). Later, dlx5a expression is observed in the perichondrium (D, black arrowheads) and in maturing chondrocytes (blue arrowheads) of proximal radials (pr) of 7.3 mm larvae. In 8.7 mm larvae, dlx5a expression is maintained in the well-developed lepidotrichia (lp) (E, orange arrowheads). Black-brown patches in B–C, E are melanophores. Scale bars B, E 20 μm, C–D 10 μm. EXPRESSION / LABELING:
|
|
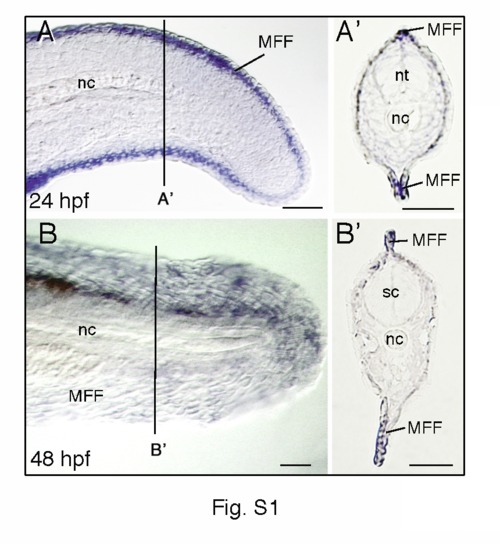
Expression patterns of dlx5a during zebrafish median fin development. Whole mount in situ hybridization for dlx5a on lateral view of the posterior axis of 24 hpf (A) and 48 hpf (B) zebrafish embryos and on 10 µm parasagittal frozen sections (A2, B2) at the level indicated in (A, B). At 24 and 48 hpf, dlx5a expression is limited to apical ectodermal cells of the median fin fold (MFF). nc, notochord; nt, neural tube; sc, spinal cord. Scale bars 50 μm. |
|
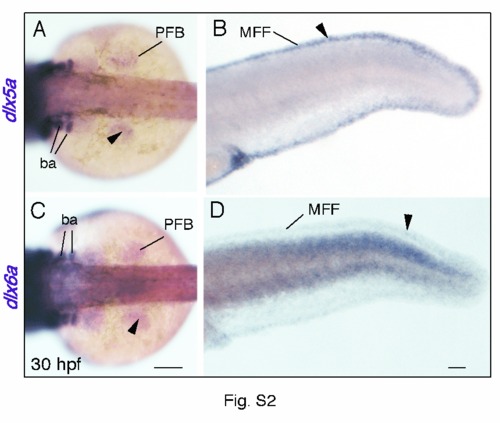
Comparison of dlx5a and dlx6a expression in the developing zebrafish fins. Whole mount in situ hybridization for dlx5a (A, B) and dlx6a (C, D) in the pectoral fin bud (PFB) (A, C) and in the median fin fold (MFF) (B, D) of 30 hpf embryos. Expression of dlx5a is detected in apical ectodermal cells of both pectoral and median developing fins (A, B, black arrowheads). Expression of dlx6a mirrors dlx5a expression, however dlx6a transcripts seem to be present at lower level (C, D, black arrowheads). Scale bars 50 μm. |
|
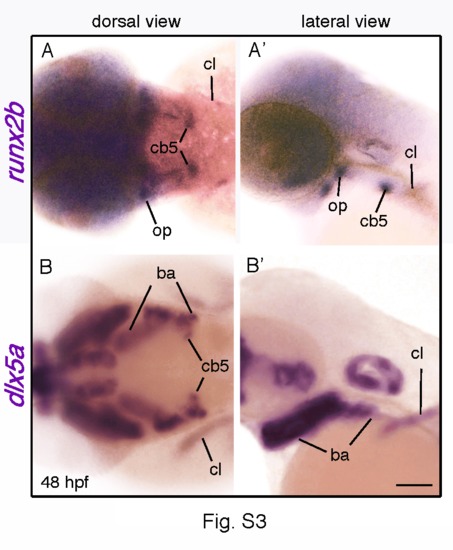
The opercle, the ceratobranchial-5 bone and the cleithrum all develop in a dlx5a-positive context. Dorsal and lateral views of whole mount in situ hybridization for runx2b (A, A2) and dlx5a (B, B2) in the anterior region of 48 hpf zebrafish embryos. Expression of runx2b reveals the opercle (op), the ceratobranchial-5 bone (cb5) and the cleithrum (cl) (A, A2), structures which differentiate at early stage of zebrafish development. The dlx5a expression analysis at equivalent stage shows that the three bones develop in dlx5a-positive domains, explaining the loss of runx2b expression in dlx5a/6a morphants at the pectoral and craniofacial levels shown in Fig. 5 D (black and blue asterisks). ba, branchial arches. Scale bars 100 μm. |
|
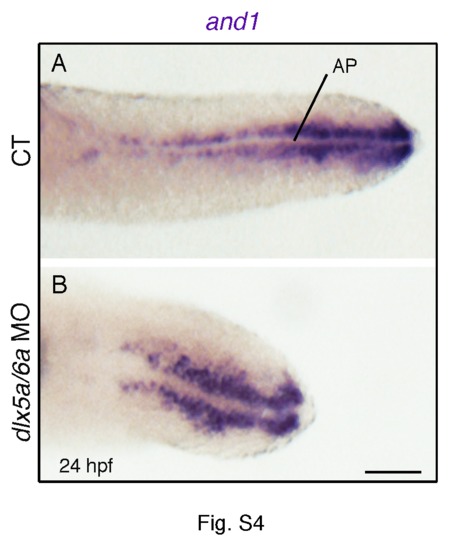
Expression of and1 in the median fin fold of control and dlx5a/6a morphant embryos. Ventral view at the posterior level of whole mount in situ hybridization for and1 in 24 hpf controls (A) and dlx5a/6a morphants (B). The ventral view reveals that and1 transcripts are not expressed in the apical ectodermal cells of the median fin fold (medial line, AP), but in ectodermal cells adjacent to the AP. Scale bar shown in B for the two panels 50 μm. |
|
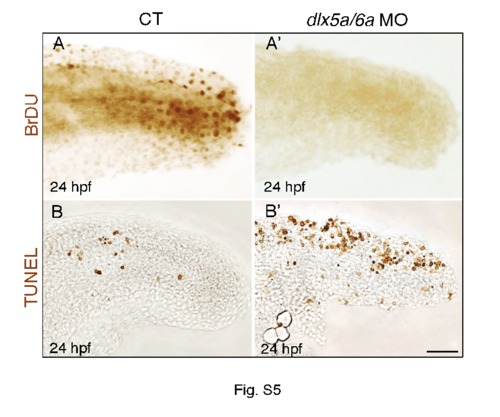
The median fin fold defects in dlx5a/6a morphants are associated with altered cell proliferation and apoptosis. Lateral view at the posterior axis of BrdU (A, A2) and TUNEL (B, B2) assays on control (A–B) and dlx5a/6a morphant (A2–B2) embryos at 24 hpf. The BrdU assay shows that controls present proliferating cells in the median fin fold whereas no BrdU-positive cells are observed in the morphants. In parallel, the morphants show a high increase of apoptotic cells in the MFF compared to controls (B–B2). Scale bar shown in B2 for all panels 20 μm. |
|
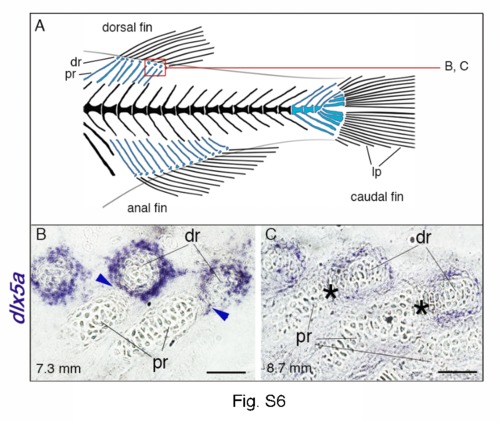
Expression of dlx5a in fin skeletal components of late-stage zebrafish larvae. (A) Overview of the posterior axial skeleton of a one-month-old zebrafish. Endoskeletal fin supports are colored blue and red square indicates the structures analyzed in (B–C). (B, C) Whole mount in situ hybridization for dlx5a on 10 µm parasagittal frozen sections of 7.3 mm and 8.7 mm late-stage zebrafish. As seen in Fig. 8 C in 6.6 mm larvae, dlx5a expression is still detected in cells surrounding the distal radials (dr) in 7.3 mm larvae, including in the zone of segmentation (ZS) (B, blue arrowheads) when the radial segmentation is almost completed. Later, after segmentation (8.7 mm), dlx5a expression is maintained but decreases in cells surrounding the distal radials and is no longer detected in the ZS (black asterisks) (C). dr, distal radials; lp, lepidotrichia; pr, proximal radials. Scale bars B–C 10 μm. |