- Title
-
Clonal evolution enhances leukemia-propagating cell frequency in T cell acute lymphoblastic leukemia through Akt/mTORC1 pathway activation
- Authors
- Blackburn, J.S., Liu, S., Wilder, J.L., Dobrinski, K.P., Lobbardi, R., Moore, F.E., Martinez, S.A., Chen, E.Y., Lee, C., Langenau, D.M.
- Source
- Full text @ Cancer Cell
|
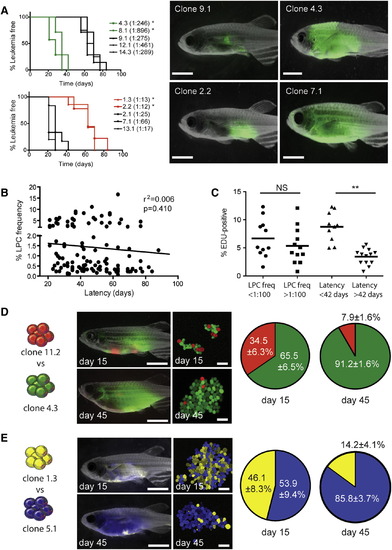
Mechanisms that Drive Leukemia Propagating Cell Frequency and Latency Can Evolve Independently (A) Fish were transplanted with 25 LPCs from various clones and assessed for time to leukemia onset (n = 8–10 animals transplanted per individual clone). Denotes significant differences in latency between clones that have low (upper left panel) or high (lower left panel) LPC frequencies (<0.001). Representative fluorescent images of animals following 28 days of engraftment are shown. (B) Correlation between LPC frequency and T-ALL latency across all clones. (C) EDU analysis of selected clones and correlation with LPC frequency and latency. Each datum point represents a single clone. NS, not significant. Denotes a significant difference in the percent of cells that are EDU-positive (p = 0.0004). (D) Animals were transplanted with 25 LPCs from clone 11.2 (dsRED-positive, 1:78 LPC frequency, 88 days latency) and 25 LPCs from clone 4.3 (GFP-positive, 1:246 LPC frequency, 28 days latency). Representative images of whole fish and confocal images of T-ALL cells harvested at 15 days and 45 days posttransplantation. The percentages of dsRED-positive and GFP-positive cells at 15 days and 45 days were analyzed by FACS. Data are represented as ± SE (n = 4–7 transplant recipients per time point). (E) Twenty-five LPCs from clone 1.3 (zsYellow-positive, 1:13 LPC frequency, 58 days latency) were competed with 25 LPCs from clone 5.1 (amCyan-positive, 1:184 LPC frequency, 30 days latency), and analyzed as in (D). Scale bars represent 5 mm in images of whole fish and 40 µm in confocal images. See also Figure S2. PHENOTYPE:
|
|
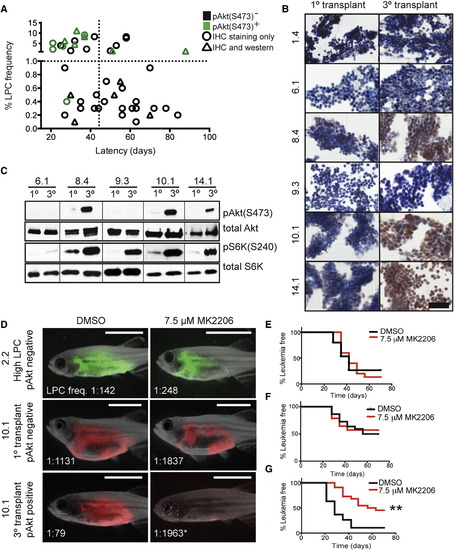
Akt Pathway Activation Is Acquired by a Subset of Cells following Clonal Evolution and Drives Elevated LPC Frequency and Growth (A) Graphical summary of pAkt(S473) IHC from 46 monoclonal T-ALL. Green denotes samples that are pAkt-positive, and black have low or absent pAkt staining. Triangles represent clones that were confirmed for pAkt status by western blot analysis. The vertical dotted line demarcates clones with short (<45 days) or long latencies, and the horizontal dotted line identifies clones with low (<1.0%) or high LPC frequency. pAkt-positivity is significantly associated with high LPC frequency (p < 0.0001) and short latency (p = 0.017) by Fisher’s exact test. (B) IHC analysis of pAkt staining in T-ALL clones. Scale bar represents 50 µm. (C) Western blot analysis of selected clones from (B). (D) Animals were transplanted with the clones indicated and treated with MK2206 or DMSO for 5 days. Representative images at 28 days posttransplantation with LPC frequencies noted. Denotes a significant change in LPC frequency following MK2206 treatment (p < 0.001). Scale bar represents 5 mm. (E–G) Kaplan-Meier analyses for T-ALL regrowth following DMSO or MK2206 treatment for clone 2.2 (E), primary monoclonal transplant clone 10.1 (F), and tertiary transplant clone 10.1 (G). Denotes a significant change in T-ALL latency following MK2206 treatment (p < 0.0001). See also Figure S4, Table S2, and Table S3. |
|
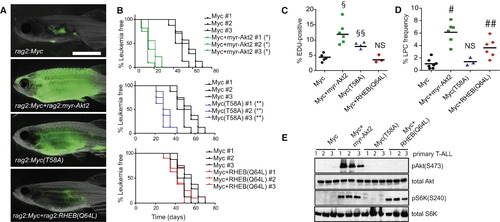
The Akt Pathway Increases LPC Frequency through Downstream Activation of mTORC1 and Shortens Latency by Augmenting Myc Stability (A) Representative images of zebrafish that were transplanted with 25 LPCs from T-ALL expressing GFP and the indicated constructs (three T-ALL per genotype, n = 35 animals transplanted per primary leukemia) at 28 days posttransplantation. (B) Kaplan-Meier analyses of time to T-ALL regrowth for each genotype and compared to Myc alone expressing T-ALL. Denotes a significant difference in latency of p < 0.0001. Indicates a significant difference in latency of p = 0.003. (C) EDU analysis of transgenic T-ALL. Each datum point represents the percent EDU-positive cells for one T-ALL. §Represents a significant difference of p < 0.0001. §§Denotes a significant difference of p = 0.004, when compared to Myc alone expressing T-ALL. NS, no significant difference. (D) Graph showing LPC frequency within each transgenic group. Each point represents data for one primary T-ALL. #Denotes a significant difference in LPC frequency of p < 0.0001. ##Indicates a significant difference in LPC frequency of p = 0.0025 when compared to Myc alone expressing T-ALL. NS, no significant difference. (E) Western blot analysis. See also Figure S5 and Table S4. PHENOTYPE:
|
|
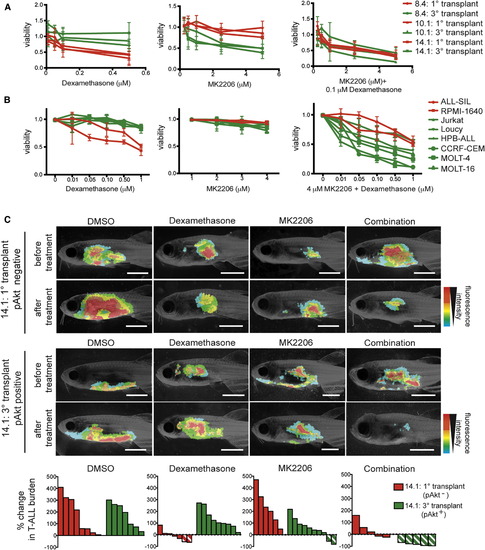
Dexamethasone Resistance Is Acquired following Akt Pathway Activation in Clonally Evolved Cells and Can Be Overcome by Combined Treatment with MK2206 (A) Primary monoclonal T-ALL that were pAkt-negative (red) and tertiary transplanted T-ALL that were pAkt-positive (green) were treated ex vivo as indicated and assessed for viability (n = 6 replicates per clone). Error bars are ± SE. (B) Human cell lines with (green) or without (red) active Akt signaling were treated in vitro as indicated. Each point is the average viability after 24 hr of drug treatment (n = 3 replicates per cell line). Error bars ± SE. (C) Representative images of leukemic fish prior to or 4 days after drug treatment with DMSO, 350 mg/l dexamethasone, 3.5 µM MK2206, or 350 mg/l dexamethasone + 3.5 µM MK2206. Clone name and pAkt status are shown to the left. Waterfall plots at the bottom summarize the in vivo T-ALL responses. Each bar denotes the percent change in T-ALL burden within a single animal, those with diagonal lines indicate >50% reduction in T-ALL burden. See also Figure S6. PHENOTYPE:
|
Reprinted from Cancer Cell, 25, Blackburn, J.S., Liu, S., Wilder, J.L., Dobrinski, K.P., Lobbardi, R., Moore, F.E., Martinez, S.A., Chen, E.Y., Lee, C., Langenau, D.M., Clonal evolution enhances leukemia-propagating cell frequency in T cell acute lymphoblastic leukemia through Akt/mTORC1 pathway activation, 366-78, Copyright (2014) with permission from Elsevier. Full text @ Cancer Cell