- Title
-
Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments
- Authors
- George, A.A., Hayden, S., Holzhausen, L.C., Ma, E.Y., Suzuki, S.C., Brockerhoff, S.E.
- Source
- Full text @ PLoS One
|
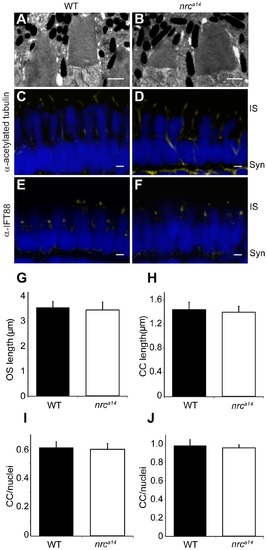
nrca14 cone photoreceptors have normal outer segments and connecting cilia. (A–B) TEM images of 5 dpf WT (A) and nrca14 (B) cone photoreceptor OSs. There was no difference in OS appearance or length (G) between nrca14 and WT cone photoreceptors at 5 dpf (p = 0.7). (C–F) Confocal images of 5 dpf zebrafish larval retinas immunostained using antibodies against the CC proteins acetylated tubulin (C, D) or IFT88 (E, F). The number (I) and length (H) of acetylated tubulin stained CC was not significantly different between WT and nrca14 photoreceptors (p = 0.9 and 0.4 respectively). There was no significant difference (p = 0.7) in the number of IFT88 stained cilia between WT and nrca14 (J). Antibody staining is shown in yellow; nuclei were stained with Sytox Green (D, E) or Hoechst (G, H) and are shown in blue. Syn = photoreceptor synapses, IS = inner segment. Scale bar = 1 μm in A and B and 2 μm in C–F. Graphs show mean values, error bars are STDEV for multiple larvae. |
|
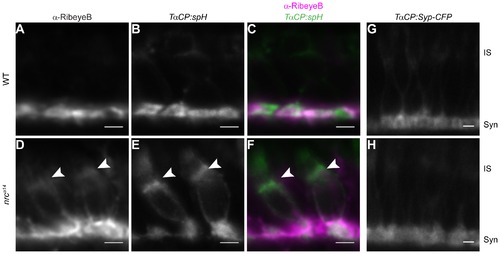
Some synaptic proteins are mislocalized in nrca14 cone photoreceptors. Tg(TαCP:spH) WT and nrca14 5 dpf retinal slices were stained with an antibody against the ribbon synapse protein RibeyeB (A–F). In WT photoreceptors, RibeyeB was found only at synaptic terminals (A). In nrca14 cone photoreceptors, RibeyeB (D, arrowhead) and VAMP2 (E, arrowhead) were detected in both synaptic terminals and ISs. Mislocalized signals for RibeyeB (magenta) and VAMP2 (green) were coincident in the ISs of nrca14 cone photoreceptors (F, arrowhead). In contrast, the synaptic vesicle protein Synaptophysin (Syp-CFP) had a primarily synaptic distribution in both WT (G) and nrca14 (H) cone photoreceptors. Live confocal images were taken of 5 dpf Tg(TαCP:Syp-CFP) WT and nrca14 larvae. Syn = photoreceptor synapses, IS = inner segment. Scale bar = 2 μm. |
|
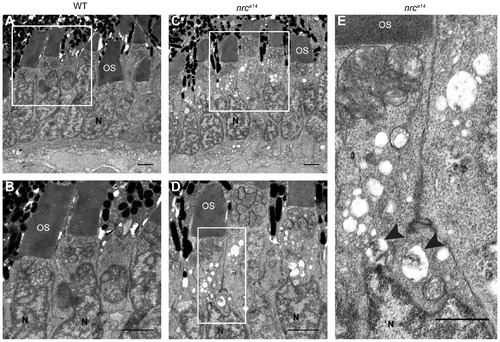
Large vesicular structures accumulate in nrca14 photoreceptor inner segments. TEM images of cone photoreceptors in WT (A, B) and nrca14 (C–E) 5 dpf retinas. Large vesicular structures were visible in approximately 80% of nrca14 cone photoreceptor ISs. Some vesicular structures contained electron dense material (arrow heads, E). Boxes in A and C show areas enlarged in B and D respectively. Box in D shows area enlarged in E. OS = outer segment, N = nuclei. Scale bar = 2 μm in A–D, 1 μm in E. PHENOTYPE:
|
|
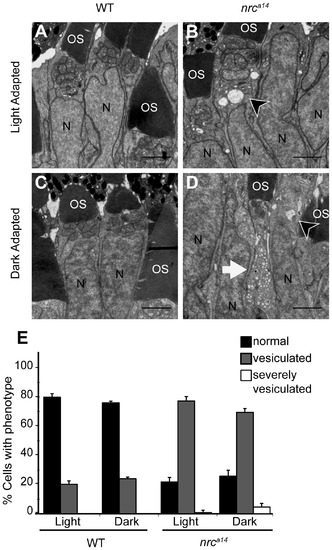
Dark adaptation increases the number of vesicular structures in nrca14 photoreceptor inner segments. TEM images of cone photoreceptors in light (A, B) or dark-adapted (C, D) WT and nrca14 retinas. At 4 dpf, larvae were phenotyped by OKR and placed at 28°C either on a normal light/dark cycle, or in complete darkness. 24 hours later, at 5 dpf, larvae were fixed for TEM. Dark adaptation exaggerated the vesicular structure phenotype in nrca14 cone photoreceptor inner segments (B vs. D). Cells were scored as “normal”, “vesiculated” or “severely vesiculated” and the quantification is shown in E. Cells with at least 3 vesicular structures >125 nm were scored as “vesiculated” and examples are shown by arrow heads in B and D, cells that contained at least 20 vesicular structures were scored as “severely vesiculated” and an example is shown by an arrow in D. OS = outer segment, N = nuclei. Scale bar = 2 μm in A–D. Graph shows mean, error bars are STDEV for three independent light/dark experiments. In total at least 400–500 cells from 5 larvae were counted per light condition and genotype. PHENOTYPE:
|
|
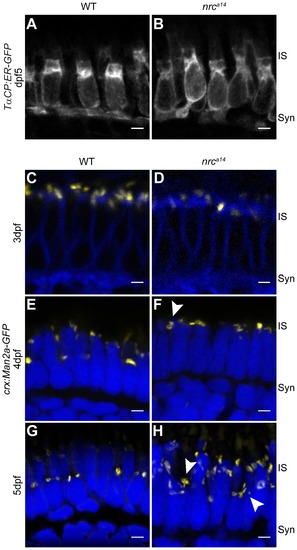
SynJ1 is required for Golgi maintenance but not development. The transgenic fish line Tg(TαCP:ER-GFP) was used to mark the ER. Retinal slices from 5 dpf WT (A) and nrca14 (B) larvae showed that the overall ER morphology was unaltered in the absence of SynJ1. Confocal images of Tg(crx:Man2a-GFP) WT (C, E, G) or nrca14 (D, F, H) larvae retina at 3–5 dpf showed that the Golgi is normal during photoreceptor development, but develops abnormalities disordered after cones become functional. No apparent abnormalities were seen in Golgi morphology of nrca14 compared to WT photoreceptors at 3 dpf (compare C and D). At 4 dpf, some mild morphology changes appeared in nrca14 Golgi (F, arrowhead). At 5 dpf, fragmented Golgi were visible in nrca14 photoreceptors (H, arrowheads). Man2a-GFP signal is shown in yellow, membranes of 3 dpf larvae were stained with BODIPY-TR and are shown in blue, and nuclei were stained with Hoechst and are shown in blue for 4 dpf and 5 dpf images. Syn = photoreceptor synapses, IS = inner segment. Scale bar = 2 μm in all images. EXPRESSION / LABELING:
PHENOTYPE:
|
|
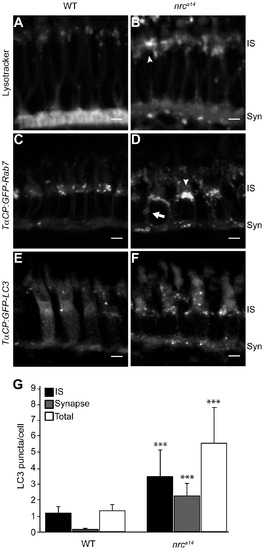
Loss of SynJ1 disrupts endolysosomal structures. Confocal images of larvae incubated with Lysotracker Red and the transgenic fish lines Tg(TαCP:GFP-Rab7) and Tg(TαCP:GFP-LC3) mark the endolysosomal system. 5 dpf larvae were incubated in Lysotracker and imaged live. In WT retinas (A), Lysotracker Red accumulated primarily in the synapse and in small punctate structures in the IS of cone photoreceptors. In nrca14 cone photoreceptors (B), Lysotracker Red accumulated primarily in larger, abnormal structures in the IS. Retinal slices from WT (C) and nrca14 (D) larvae show that abnormal Rab7-positive structures including large perinuclear structures (arrowheads), and doughnut shaped structures (arrows) accumulated in the IS and synapse in the absence of SynJ1. The lack of SynJ1 also caused an increase in the number of LC3-GFP-positive structures, indicating a disruption in autophagy in nrca14 cones (F). The number of LC3 puncta is increased in both the IS and synapse of nrca14 cones (G). Syn = photoreceptor synapses, IS = inner segment. Scale bar = 2 μm in all images. Graph shows average LC3 puncta in the IS, synapse or entire cell (Total) per cell, error bars are STDEV for 11 WT larvae and 12 nrca14 larvae. The number of LC3 puncta per cell for each subcellular compartment or the entire cell was significantly different between WT and nrca14 larvae (p-value<0.001, donated by ***). |
|
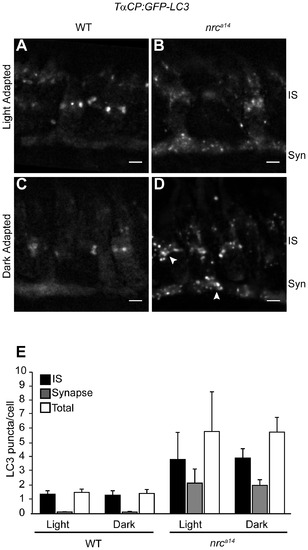
Dark adaptation affects autophagosomes in nrca14 cone photoreceptors. Confocal images of cone photoreceptors in light (A, B) or dark-adapted (C, D) WT and nrca14 Tg(TαCP:GFP-LC3) retinas. At 4 dpf, larvae were phenotyped by OKR and placed at 28°C either on a normal light/dark cycle, or in complete darkness. 24 hours later, at 5 dpf, larvae were fixed and retinal slices were generated. After dark incubation, the GFP-LC3 positive puncta in nrca14 cone photoreceptors appeared enlarged (D, arrowhead). Dark incubation did not significantly affect the number of GFP-LC3 puncta in WT or nrca14 cone photoreceptors (E). Syn = photoreceptor synapses, IS = inner segment. Scale bar = 2 μm in all images. Graph shows average LC3 puncta in the IS, synapse or entire cell (Total) per cell, error bars are STDEV, from two independent light/dark experiments. For each experiment, 3–5 larvae were used per condition, and 60–150 cells were analyzed per larvae. |
|
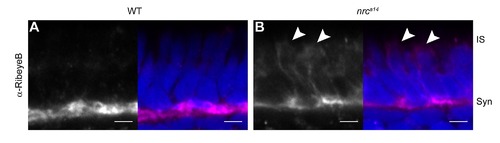
RibeyeB is mislocalized in nrca14 inner segments even in the absence of TαCP:spH. Anti-RibeyeB staining of non-transgenic WT and nrca14 5 dpf retinas showed the same staining pattern as Tg(TαCP:spH) 5 dpf retinas in Figure 2. In WT photoreceptors, RibeyeB was found only at synaptic terminals (A). In nrca14 cone photoreceptors, the RibeyeB staining was visible in both the synaptic terminals and ISs (B). Anti-RibeyeB staining is shown in magenta and Hoechst stained nuclei are in blue. Syn = photoreceptor synapses, IS = inner segment. Scale bar = 2 μm in all images. |
|
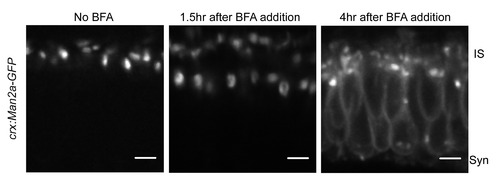
Man2a-GFP marks medial Golgi structures. We confirmed that our Golgi marker behaved in the same manner as endogenous Golgi proteins by treating 3 dpf Tg(crx:Man2a-GFP) zebrafish larvae with 2 μM Brefeldin A followed by live imaging. After a 4 hour incubation in BFA, the GFP signal was present in the ER and fragmented Golgi structures. |