- Title
-
Characterization of light lesion paradigms and optical coherence tomography as tools to study adult retina regeneration in zebrafish
- Authors
- Weber, A., Hochmann, S., Cimalla, P., Gärtner, M., Kuscha, V., Hans, S., Geffarth, M., Kaslin, J., Koch, E., and Brand, M.
- Source
- Full text @ PLoS One
|
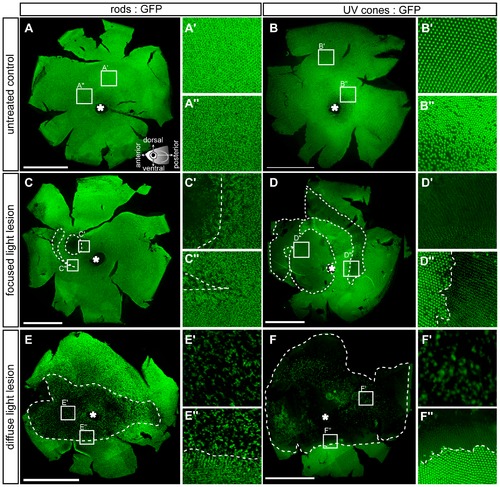
Flatmounted retina samples of lesioned GFP transgenic reporter fish. Rods (left column) are labelled with the rh1:GFP and UV cones (right column) with the opn1sw1:GFP reporter fish, respectively. A, B: Vitreal view of untreated control eyes. Insets show magnified images indicated in the overviews. Illustration showing the orientation of flatmounted samples as vitreal view with dorsal (superior) orientation to the top. C-C3: 3 days post focused light lesions in rh1:GFP. D-D3: 3 days post focused light lesions in opn1sw1:GFP. E-E3: 3 days post diffuse light lesions in rh1:GFP. F-F3: 3 days post diffuse light lesions in opn1sw1:GFP. The asterisk in A–F marks the optic nerve head. Scale bars represent 1 mm. |
|
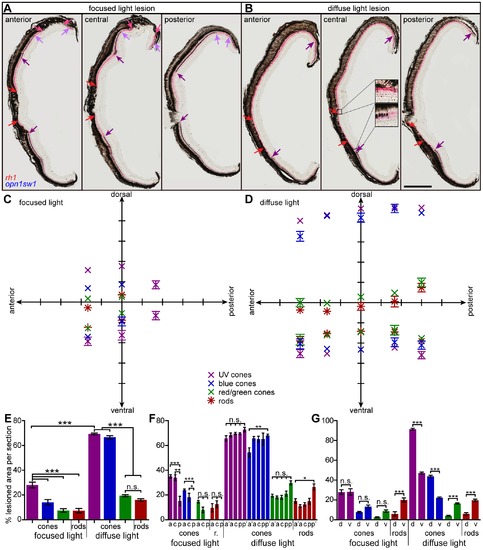
Extent of light lesioned areas in anterior-posterior and dorsal-ventral comparison. A, B: Double in situ hybridization against rhodopsin (red) to label rods and opn1sw1 (blue) to label UV cones. A: Focused light lesioned retinas in an anterior (left), central (middle) and posterior section (right). Central UV cone lesions are labeled with violet arrows, rod lesions are labeled with red arrows. Light purple and pink arrows indicate peripheral lesions of UV cones and rods, respectively. B: Diffuse light lesions analogous to A. Insets in the central section show the transition from healthy to lesioned areas. C: Blot of lesioned areas relative to their location in the retina. The average of dorsal and ventral lesion boundaries are blotted in the respective color code indicated in the legend. D: Lesioned areas analogous to C for diffuse light paradigm with additional far anterior and far posterior measure points. E: Comparison of average total damaged area in % of total retina length. F: Comparison of average anterior vs. posterior damaged area in % of total retina length. G: Comparison of average dorsal vs. ventral damaged area in % of 1/2 retina length. a: anterior, a2: far anterior, c: central, d: dorsal, p: posterior, p2: far posterior, r: rods; v: ventral. Column colors indicate cone type as in the legend (C). Error bars indicate standard error of the mean; *** for p-values <0.001; ** for p-values <0.01; * for p<0.05; n.s.: not significant; scale bar represents 400 μm. |
|
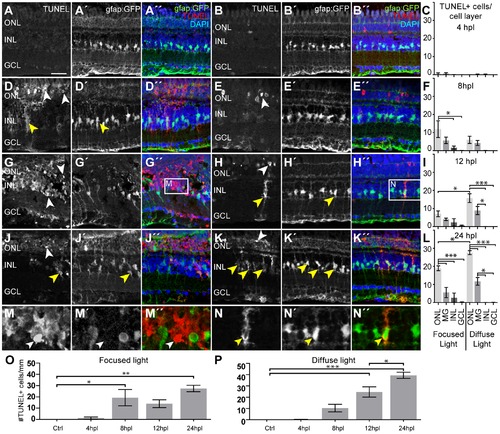
Cell death after light lesions in Tg(gfap:GFP) fish. TUNEL+ cells are labeled in red, MG are GFP+ (green), nuclei are stained with DAPI (blue). Quantification shows TUNEL+ cells per cell layer normalized to 1 mm retinal length in (C, F, I, L). GFP/TUNEL double positive cells are indicated as ‘MG’ to discriminate between phagocytic MG and real INL cell death. A–C: Only very few TUNEL+ cells were found early at 4 hpl. D–I: Increasing amounts of TUNEL+ cells were found between 8 hpl and 12 hpl (white arrowheads). The density of TUNEL+ cells is high in the center of focused light lesions but lower along the whole retina after diffuse light lesions. J–L: A peak of TUNEL+ cells is found at 24 hpl in both lesion paradigms. M: Many TUNEL+ INL cells that are GFP- (white arrowhead) were found in the centre of focused light lesions, but were rare elsewhere. N: Example of TUNEL+ MG. TUNEL+ signal is found in the cytoplasm (yellow arrowhead). O, P: Time course of the total number of TUNEL+ cells per mm peaking at 24hpl. dpl: days post lesion; GCL: ganglion cell layer; hpl: hours post lesion; INL: inner nuclear layer; MG: Müller glia; ONL: outer nuclear layer; Error bars indicate standard error of the mean; *** for p-values <0.001; ** for p-values <0.01; * for p<0.05; scale bars represent 20 μm. |
|
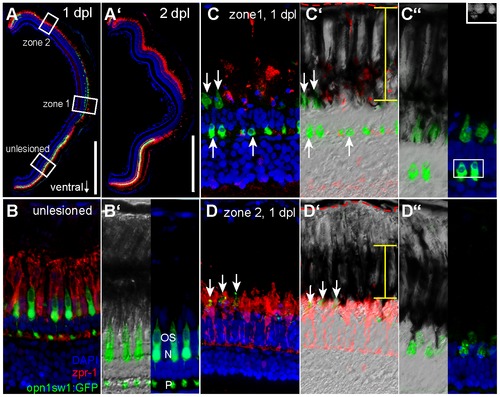
Differential removal of dying UV cones in zone 1 and 2. A: Overview image of a retinal section from an opn1sw1:GFP transgenic animal at 1 and 2 days post diffuse light lesion (A2). B: Unlesioned red/green double cones are labelled by zpr-1 (red) and UV cones by GFP. Nuclei were stained with DAPI (blue). B2: Close up of outer segments (OS), nucleus (N) and pedicle (P) is in the OPL. C: Central retina (zone 1) showing removal of zpr1+ cones while disrupted UV cones persist (arrow). C2: DIC image showing the distribution of pigmented granula in the RPE (yellow bar) relative to Bruch′s membrane (red dashed line). C3: Inset shows pyknotic nuclei in DAPI channel. D: Peripheral lesion with intact red/green cones but depleted UV cones. D2: DIC image showing condensed RPE pigments. D3: Co-localisation of pigmented processes with remaining GFP debris. ONL: Outer Nuclear Layer; RPE: Retina Pigmented Epithelium. Scale bars represent 500 μm. |
|
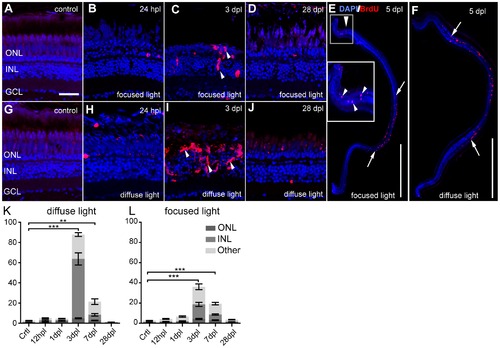
Proliferative response after light lesions. Proliferating cells are labelled by BrdU (red). A, G: Untreated control shows weak autofluorescence in photoreceptors. B–D: Clusters of BrdU+ cells are indicated by white arrowheads. E–F: Overview images showing proliferating cells at 5 dpl in the characteristic lesion areas (between arrows). G–J: Time course after a diffuse light lesion. K, L: Quantification of BrdU+ cells in the whole retina per cell layer normalized to 1 mm retinal length. Most proliferating cells are found in the INL and ONL at 3 dpl. BrdU+ cells found in the ganglion cell layer and the ciliary marginal zones are indicated as ‘Other’. Error bars indicate SEM. Scale bar represents 20 μm (A–D, G–J) or 500 μm (E, F). |
|
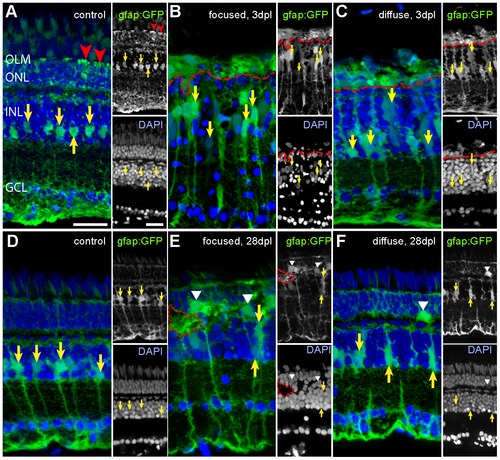
Müller glia cells after light lesions, labelled by the gfap:GFP reporter line (green) and DAPI (blue). Somata and processes of MG are indicated by yellow and red arrows, respectively. A: Untreated control retina. MG processes in the ONL are indicated by red arrowheads. B: 3 days after focused light lesion in the lesion centre. Swollen MG nuclei were found (yellow arrows). MG processes in the ONL collapsed (above dashed red line). C: 3 days after diffuse light lesions. ONL processes of MG collapsed (above dashed red line). D: Control side of focused light treated fish after 28 dpl. E: 28 days after focused light lesion in the lesion centre. Somata of MG are larger and sometimes displaced to the ONL (white arrowhead). Gaps in DAPI channel (dashed red line) are filled with GFP+ MG. F: 28 dpl after diffuse light lesion. Some displaced MG were found in the ONL (white arrowhead). |
|
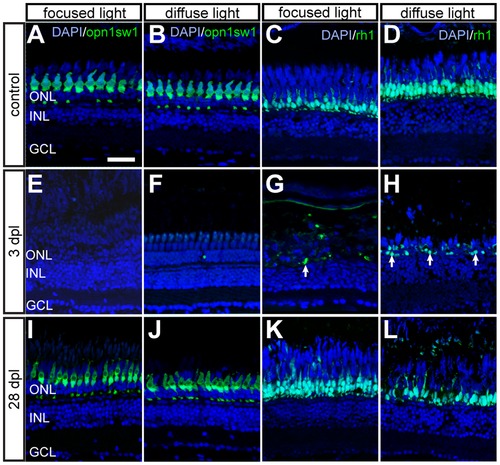
Time course of GFP+ photoreceptors after light lesions. UV cones (left panel) and rods (right panel) are labelled by the opn1sw1:GFP and rh1:GFP reporter line, respectively. A, B: Untreated control showing continuous mosaic pattern of UV cones. C, D: Untreated control showing the continuous rod layer in the ONL. E, F: Loss of all GFP+ cones 3 days after focused and diffuse light lesions. G, H: Partial depletion of rods 3 dpl after focused and diffuse light lesions. Examples of remaining rods are indicated by arrows. I, J: Regenerated UV cones in the ONL at 28 days after focused and diffuse light lesions. K, L: GFP+ rods regenerate in the appropriate layer after focused and diffuse light lesion. Scale bars represent 20 μm. |
|
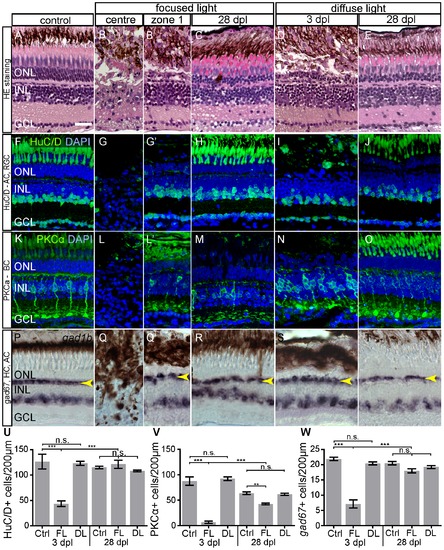
Morphology and Immunohistochemistry of neuronal cell types in light lesions at 3 and 28 dpl. A–E: Light lesioned retina morphology shown by hematoxylin/eosin staining. Severe damage to all laminae is found in the centre of focused light lesion zone 1 in an area of ~50 μm in diameter. F–J: HuC/D antibody staining labels nuclei of AC and RGC. K–O: Antibody-staining against Phospho-Kinase C α-subunit (PKCα) labels a subset of bipolar cells. P–T: HC and a subset of AC are labelled by gad1b in situ hybridization. The continuous line pattern of HC is indicated by the arrowhead. U–W: Quantification of inner retinal neurons. The bars indicate the number of marker+ cells in 200 μm length of the retina across the most severe lesion. As control served untreated eyes of focused light treated fish. Regeneration of all cell types was assessed at 28 dpl. Error bars indicate SEM; ***p<0.001; AC: amacrine cells, BC: bipolar cells; DL: diffuse light lesions; FL: focused light lesions; GCL: ganglion cell layer; HC: horizontal cells; INL: inner nuclear layer; PR: photoreceptor cells; RGC: retinal ganglion cells. Scale bar represents 20 μm. |
|
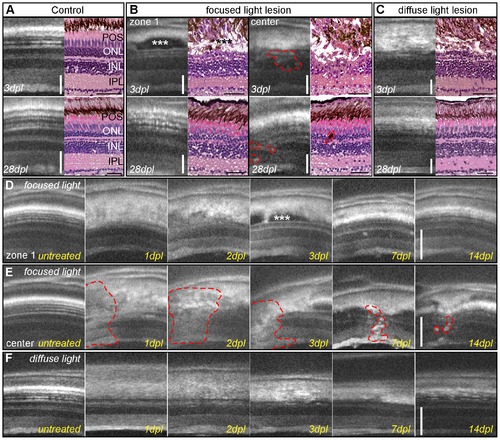
Imaging of light lesions with SD-OCT. A: Comparison of OCT images to HE staining, showing nuclear layers in dark shades and plexiform layers in grey. RPE cells appear as a thick white band over the thinner white spots of POS. B: Focused light lesions show strong heterogeneity, at least two different morphological areas are found in OCT and HE: zone 1 (left panel) with an intact inner nuclear layer, elongated nuclei (arrow) and accumulation of non-light-scattering material between ONL and RPE (asterisk). The center of focused light lesions (right panel) shows disorganization in all retinal layers between GCL and RPE (dashed line). Regenerated retina showing original thickness and morphology in zone 1 (left) but small inclusions in the center of focused light lesion (right) C: Homogeneous lesion at 3 days after diffuse light lesions in OCT and HE staining with a characteristic loss of ONL. Regenerated retinas closely resemble the morphology of control samples. D, E: Time course of the two typical morphologies after focused light lesion: the accumulation of material in zone 1 (3 dpl, asterisk) was cleared away later, whereas the center still shows small inclusions and flaws compared to the original structure (E, dashed line.) F: Time course of diffuse light lesions showing disorganization of POS and RPE at 1 and 2 dpl followed by a loss of the ONL at 3 dpl and regeneration of ONL and POS at 14 dpl. GCL: ganglion cell layer; HE: hematoxylin/eosin; INL: inner nuclear layer; IPL: inner plexiform layer; ONL: outer nuclear layer; POS: photoreceptor outer segments; RPE: retinal pigmented epithelium; SD-OCT: spectral domain optic coherence tomography. Scale bar represents 50 μm. |
|
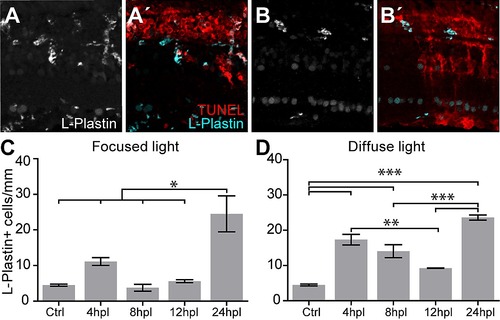
L-Plastin+ cells are enriched in light lesions at 24 hpl. A: L-Plastin+ cells were found in various retinal cell layers after focused light lesions. A2: Double labeling with TUNEL (red) shows only minimum overlap and mostly complementary location of L-Plastin+ cells (cyan). B, B2: Diffuse light lesion analogous to A, A2. C, D: Time course of absolute number of L-Plastin+ cells found in focused (C) and diffuse light lesion (D) showing a peak at 24 hpl. Error bars indicate standard error of the mean; *** for p-values <0.001; ** for p-values <0.01; * for p<0.05. |
|
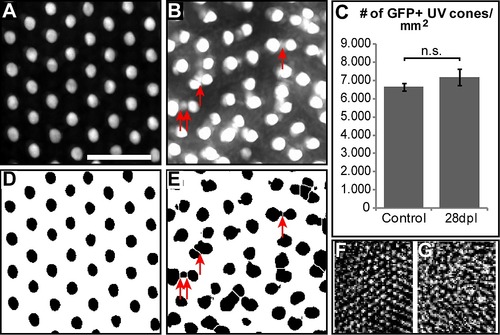
Changes in UV cone mosaic after regeneration in light lesions. A: Close-up image of untreated control retina flatmount from opn1sw1:GFP fish. B: Close up image of regenerated retina (28 dpl) from the same line. C: Quantification of UV cones per area compared (n = 6; p = 0.28; error bars indicate SEM). D, E: Same as A, B after image modification in order to count the number of cones automatically with Fiji software. Scale bar represents 20 μm. F, G. In vivo image of UV cones from OCT Data before (F) and at 28 dpl after light lesion (G) from the same fish. |
|
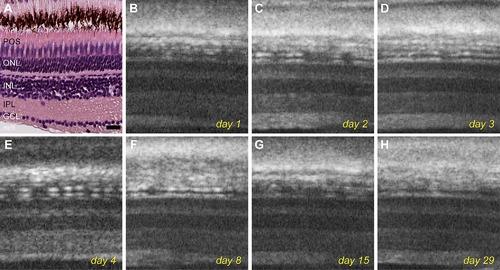
Live imaging of an untreated control fish over the course of 29 days. A: Histological staining of an untreated retina shows typical retinal layer structure. B–H: OCT images of the same fish acquired over 1 month shows no change in retinal structures. Scale bar represents 20 μm. |