- Title
-
Retinoic Acid metabolic genes, meiosis, and gonadal sex differentiation in zebrafish
- Authors
- Rodríguez-Marí, A., Cañestro, C., Bremiller, R.A., Catchen, J.M., Yan, Y.L., and Postlethwait, J.H.
- Source
- Full text @ PLoS One
|
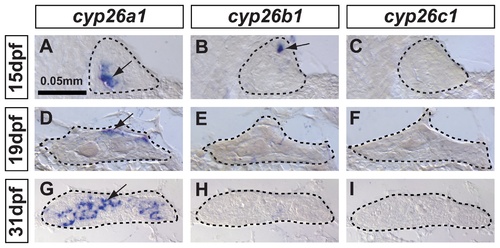
In zebrafish, cyp26a1 is the main Cyp26 paralog expressed in gonads during the critical period for sex-determination. Undifferentiated gonads at 15 days post-fertilization (dpf) expressed cyp26a1 (A) and cyp26b1 (B), but did not express cyp26c1 at detectable levels (C) (A-C: n=4). In bipotential gonads at 19 dpf, cyp26a1 expression became restricted mainly to the dorsal margin of the gonad (D) but expression of cyp26b1 and cyp26c1 was not detected (E, F) (D–F: n=9). In differentiating testes at 31 dpf, cyp26a1 expression up-regulated (G) and in contrast to mouse testes, which up-regulate cyp26b1, neither cyp26b1 nor cyp26c1 expression was detected in maturing zebrafish gonads (H, I) (G-I: n=10). Differentiating testes were assigned by morphological features and assessed by the expression of the male specific Amh marker (see Figure 4). We conclude that in zebrafish, Cyp26a1 is expressed at the time and place necessary to provide an RA-degrading function equivalent to Cyp26b1 in tetrapods. These results suggest independent subfunction partitioning of ancestral cyp26 regulatory elements in lineages leading to zebrafish and mouse. Arrows point to examples of expressing cells. Dashed lines outline gonads. Scale bar: 0.05mm. EXPRESSION / LABELING:
|
|
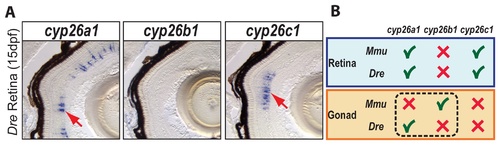
Expression patterns of cyp26 gene family members in late developing retina are conserved between zebrafish and mouse. To learn if the divergent subfunction partitioning between zebrafish cyp26a1 and mouse cyp26b1 in gonad development applies to other organs, we studied the expression of cyp26 paralogs during retina development in 15-dpf zebrafish. Results revealed expression of cyp26a1 and cyp26c1 in different layers of the retina, but no detectable expression of cyp26b1 (A: n=1). This result agrees with the Cyp26 expression profile described in mouse retina (B), in which Cyp26a1 and Cyp26c1 genes were expressed in the inner nuclear layer during post-natal eye development while Cyp26b1 was not expressed in the eye [74]. We conclude that independent subfunction partitioning related to gonad development occurred after the teleost-tetrapod lineage divergence, while subfunctions related to at least one other organ, the retina, are still conserved between Cyp26 orthologs in different vertebrate lineages (B). EXPRESSION / LABELING:
|
|
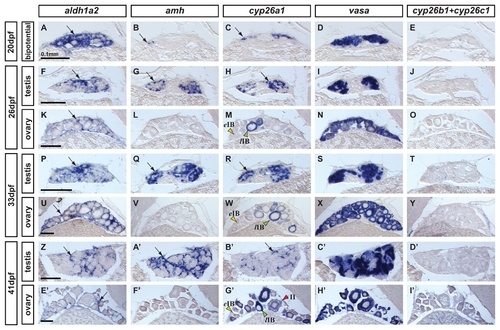
Expression of genes encoding enzymes for the synthesis and degradation of RA during zebrafish gonad development. In situ hybridization on adjacent sections of animals representing the three key stages of gonad development: (A–E: n=7) bipotential, sexually undifferentiated gonads with an ovary-like morphology at 20 days post-fertilization (dpf); (F–O) gonads transitioning to testes or ovaries (26 dpf) (F-J: n=4; K–O: n=3); (P–Y and Z–I′) gonads sexually differentiated but still immature (33 dpf and 41 dpf) (P-T: n=3; U–Y: n=4; Z-D′: n=2; E′-I′: n=2). Images show expression patterns of the gene encoding the RA-synthesizing enzyme Aldh1a2 (A, F, K, P, U, Z, E′), the gene encoding the RA-degrading enzyme Cyp26a1 (C, H, M, R, W, B′,G′) and combined probes for cyp26b1 and cyp26c1 (E, J, O, T, Y, D′, I′) together with the early male gonadal marker amh (anti-Müllerian hormone: B, G, L, Q, V & A′, F′) and the germ-line specific marker vasa (D, I, N, S, X, C′, H′) at four different stages. Expression of aldh1a2 was detected in somatic cells in both male and female gonads throughout development (A, F, K, P, U, Z, E′). Expression of aldh1a3 was not detected at all in gonads but was detected in retina cells (data not shown) and the ortholog of Aldh1a1 was lost in the teleost lineage [54-56]. The expression pattern of cyp26a1, however, showed a distinct sexual dimorphism, as gonadal somatic cells from males (H, R and B′), but not from females (M, W, G′), up-regulated its expression during gonad development. Interestingly, in females, oocytes that had transitioned from early stage IB (yellow arrowhead in M,W,G′) to late stage IB (green arrowheads in M,W,G′) up-regulated the expression of cyp26a1 in the ooplasm, which was maintained at later stages (e.g. red arrowhead stage II in G′). The observed expression pattern of cyp26a1 in oocytes is compatible with a function in inhibiting meiotic progression and facilitating the meiotic arrest at diplotene stage. Expression of cyp26b1 and cyp26c1 in gonads was not detected at any of the stages analyzed in either sex (E, J, O, T, Y, D′, I′) Arrows point to examples of expressing cells. Scale bar shown per each row: 0.1mm. EXPRESSION / LABELING:
|
|
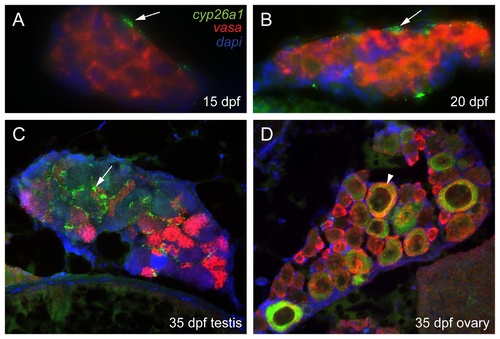
Three color fluorescent detection of cyp26a1 and vasa expression during zebrafish gonad development. Fluorescence detection of cyp26a1 (green) expression and vasa (red) expression by double in situ hybridization on gonad sections at the bipotential gonad stage at 15 dpf (A: n=1) and 20 dpf (B: n=2), and on immature gonads developing into testes (C: n=1) or ovaries (D: n=1) at 33 dpf. Cyp26a1 expression occurs in somatic cells and does not co-localize with the germ cell marker vasa in bipotential gonads (A,B). In immature testes, cyp26a1 expression was also expressed in somatic cells (arrow) and not in germ cells (C). In immature ovaries, however, cyp26a1 was expressed in large oocytes that had reached diplotene (arrowhead in D). EXPRESSION / LABELING:
|
|
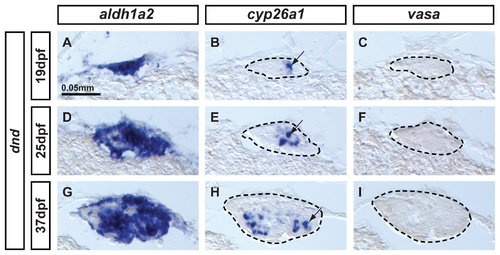
Expression of aldh1a2 and cyp26a1 is independent of germ cell signaling. Animals depleted of germ cells that develop into sterile males were generated by dead end (dnd) morpholino knockdown to study the expression of aldh1a2 and cyp26a1 in gonads at (A-C: n=1) bipotential stage (19dpf), (D–F: n=1) transitioning stage (25dpf), and (G-I: n=1) differentiated testes (37dpf). Results showed that aldh1a2 was widely expressed in somatic cells of the gonads at the three different stages analyzed (A,D,G) while cyp26a1 expression was detected in a subset of somatic cells also in the three stages analyzed (arrows in B, E, H). These results demonstrate that the onset as well as the maintenance of aldh1a2 and cyp26a1 expression, at least until 37dpf, is independent of germ cell signaling. Expression of the germ cell specific marker vasa was not detected at any stage (C,F,I), confirming the total depletion of germ cells by dnd morpholino injection in all animals. Gonads are outlined by a dashed line (B,C, E, F,H,I). Scale bar: 0.05 mm (A). EXPRESSION / LABELING:
|
|
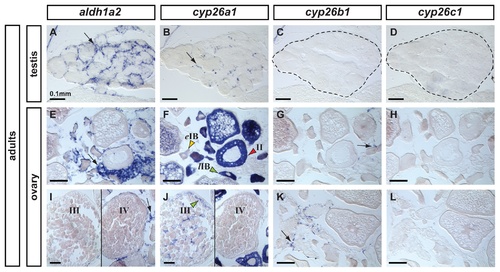
Expression patterns of aldh1a2, cyp26a1, cyp26b1 and cyp26c1 in adult gonads. In adult testes (n=3), aldh1a2 expression was detected in somatic cells surrounding cysts in a localization expected for Sertoli cells (A) and cyp26a1 expression was detected in a subset of cells in a localization expected for Leydig cells (B). The presence of germ cells expressing cyp26a1 could not be discarded. Expression of cyp26b1 (C) and cyp26c1 (D) was not detected in adult testes. In adult ovaries (n=2), aldh1a2 was detected in somatic cells but not in the oocytes (E,I). Expression of cyp26a1 was restricted to the ooplasm of oocytes and was not detected in somatic cells (F,J). In oocytes, cyp26a1 expression varied according to the stage of meiosis: it was barely detectable in early stage IB oocytes (eIB, yellow arrowhead in F, prior to the diplotene stage of meiosis), was up-regulated in late stage IB oocytes that entered meiotic arrest at diplotene stage (lIB, green arrowhead in F), was maintained in stage II (red arrowhead in F) and stage III oocytes (green arrowhead in J) and was not detected in stage IV oocytes (J) coinciding with the resumption of meiosis I. Expression of cyp26b1 was detected solely in a subset of cells in the somatic tissue surrounding the oocytes (arrows in G, K) and no expression of cyp26c1 was detected in the ovary (H,L). Scale bar: 0.1mm. EXPRESSION / LABELING:
|
|
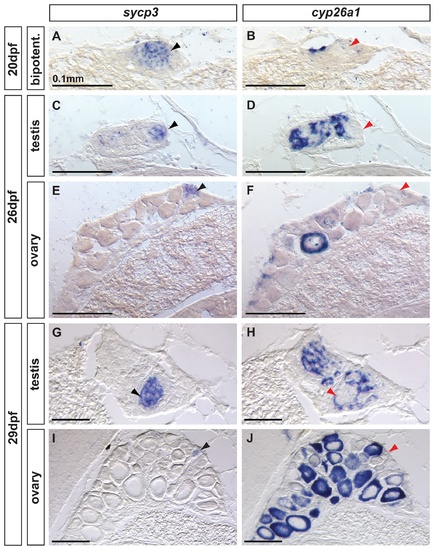
Complementary expression of the meiotic recombination marker sycp3 and cyp26a1 in developing gonads. In bipotential gonads at 20 dpf (A,B: n=8), germ cells expressed the meiotic recombination marker sycp3 (black arrowhead in A) in a non-overlapping pattern with cyp26a1 expression, which was mostly restricted to the dorsal part of the gonad (revealing that sycp3-expressing cells did not express cyp26a1 (red arrowhead in B). Expression of the meiotic marker sycp3 was detected in bipotential gonads of all animals analyzed (A, n=8) suggesting that some germ cells entered meiosis in all juveniles regardless of their definitive sex. In differentiating testes at 26 dpf (C,D: n=2) and 29 dpf (G, H: n=2), islands of germ cells that expressed sycp3 (black arrowheads in C, G) were found in an area in which RA was likely not degraded due to lack of cyp26a1 expression (red arrowheads in D,H). In contrast, in differentiating ovaries at 26 dpf (E, F: n=2) and 29 dpf (I, J: n=2), sycp3 was expressed in small germ cells (black arrowheads in E, I) that did not express cyp26a1 (red arrowheads in F, J). The expression of cyp26a1 was restricted to the ooplasm of oocytes that reached diplotene stage and entered in meiotic arrest (F,J). Scale bar: 0.1mm. EXPRESSION / LABELING:
|
|
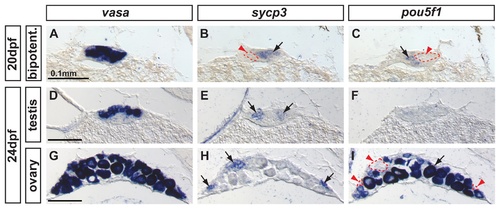
Complementary expression of the meiotic recombination marker sycp3 and the pluripotent marker pou5f1(oct4) in developing gonads. In bipotential gonads at 20 dpf, comparison of the expression of the germ cell marker vasa (A), the synaptonemal complex marker sycp3 (arrow in B) and the pluripotent gene pou5f1 (arrow in C) revealed that sycp3 and pou5f1 were both expressed in germ cells but in a complementary non-overlapping fashion (red arrowheads and dashed lines in B and C). In differentiating testes at 24 dpf, expression of vasa was detected in germ cells (D) revealing that only some of the germ cells expressed sycp3 (arrows in E) but none of them expressed pou5f1 (F). In differentiating ovaries at 24 dpf, vasa labeled germ cells (G), and sycp3 only labeled those germ cells that were small (arrows in H), which interestingly did not express pou5f1 (red arrowheads and dashed lines in I) and had not reached the late stage IB. Complementarily, the larger oocytes that had reached diplotene stage expressed pou5f1 (arrow in I). Scale bar indicated per each raw: 0.1mm. EXPRESSION / LABELING:
|
|
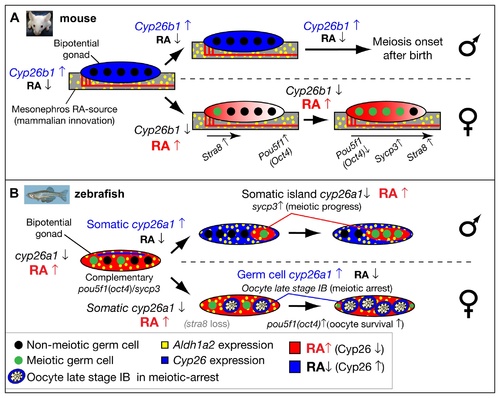
Model for the role of retinoic acid and meiotic progression during gonadogenesis in mouse and zebrafish. In mouse (A), Aldh1a2 (yellow) in the mesonephros provides the RA-source that regulates gonad development (reviewed in [18]), while in zebrafish (B), aldh1a2 is expressed by somatic cells within the gonad (yellow), and thereby provides an internal RA-source. In mouse, non-meiotic germ cells (black circles) in bipotential gonads are protected from RA by high expression of Cyp26b1 (blue), while in zebrafish, Cyp26a1 expression (blue) is restricted to cells at the dorsal surface near the body cavity, and thereby germ cells elsewhere in the gonad are not protected from RA (red) and they are able to enter into meiosis (green circles). In mouse, sexually dimorphic expression of Cyp26b1 causes low levels of RA (blue) in testes (males, top in A) and high levels of RA (red) that diffuses from the mesonephros to the ovaries in an anterior to posterior wave (females, bottom in A), which results in a sexually dimorphic onset of meiosis. The onset of meiosis in mouse follows an anterior–posterior wave, accompanied by up-regulation of Stra8, and Sycp3 and down-regulation of Pou5f1(Oct4) [18]. In zebrafish, the sexually dimorphic expression of cyp26a1 differs in time and location from those of Cyp26b1 in mouse. In zebrafish males (top in B), somatic cells up-regulate cyp26a1, and meiotic cells expressing sycp3 localize to somatic islands (red) that lack cyp26a1 expression. This model is consistent with a role for Cyp26a1 in degrading RA and thereby protecting nearby germ cells from progressing through meiosis. Consistent with this model, in females (bottom in B), cyp26a1 is not expressed in somatic cells, but it is up-regulated in the ooplasm (blue) of oocytes at late stage IB that have reached diplotene stage and have entered in meiotic arrest, and also express pou5f1(oct4). The co-expression of cyp26a1 and pou5f1(oct4) is compatible with the proposed roles of these genes in mouse on promoting germ cell survival and preventing apoptosis [41, 86], mechanisms that have been shown to be central for tipping the sexual fate of the gonad toward the female pathway in zebrafish [ 5, 8, 87]. |

Unillustrated author statements EXPRESSION / LABELING:
|