- Title
-
Moving domain computational fluid dynamics to interface with an embryonic model of cardiac morphogenesis
- Authors
- Lee, J., Moghadam, M.E., Kung, E., Cao, H., Beebe, T., Miller, Y., Roman, B.L., Lien, C.L., Chi, N.C., Marsden, A.L., and Hsiai, T.K.
- Source
- Full text @ PLoS One
|
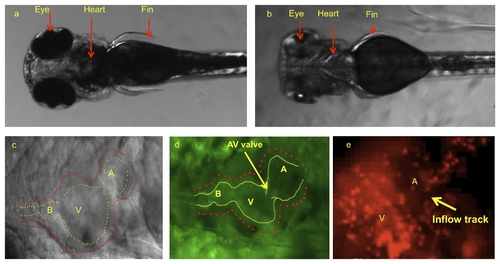
Dorsal view of zebrafish embryos illustrated cardiac system prior to and after pigmentation removal. (a) Pigmentation opacifies internal organ systems at 120 hpf. (b) Treatments with PTU beginning at 10 hpf prevented pigment formation, allowing for clear organ visualization. (c) PTU-treated zebrafish heart observed under bright field microscope at 120 hpf. Despite visualization of outer wall, inner wall is poorly demarcated. (d) The use of transgenic Tg(fli1a:EGFP)y1 embryos allows for clear delineation of inner wall for reconstructing the CFD model. (e) Tg(gata1:dsRed)sd2 transgenic zebrafish allows to visualize the blood particle in order to take velocity by particle tracking technique. A, atrium; V, ventricle; B, bulbus arteriosus. |
|
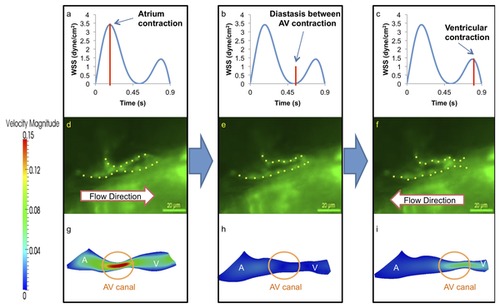
At 20-30 hpf, zebrafish heart is morphologically a tube-like structure with a AV canal (AV) separating the atrium (A) and ventricle (V). (a) Atrial contraction, the first peak, engenders an increase in shear stress profiles through the AV canal. (b) Diastasis between atrial and ventricular contraction result in a decrease in shear stress across the AV canal. (c) Ventricular contraction, the second peak, results in flow reversal or regurgitation through the AV canal. The magnitude of wall shear stress (WSS) across the AV canal is about half of the forward blood flow at AV canal. (d) The green fluorescent protein (GFP) delineated the tubular structure. Unidirectional forward flow from the contracting atrium into the ventricle develops through the AV canal. (e) The GFP image reveals movement of peristaltic wave to the end of tubular heart structure. (f) Flow reversal develops in response to the contraction of tubular structure. (g) Velocity profiles show the maximal magnitude through the AV canal. (h) The magnitude of velocity is minimal at AV canal. (i) The magnitude of flow reversal or regurgitation is about half of the forward blood flow at AV canal. Contoured velocity profiles confirm the observed result (g–i). Red bars in (a–c) represent the time points at which the corresponding velocity profiles were reconstructed. A, atrium; V, ventricle. |
|
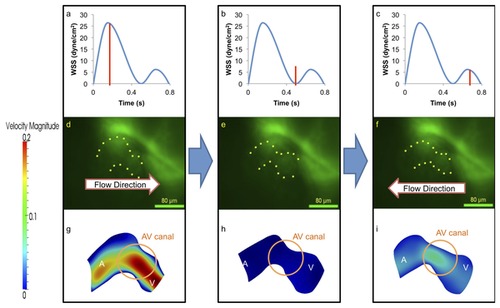
At 40-50 hpf, the zebrafish heart begins to undergo looping. (a–c) Compared to 20-30 hpf, WSS across the AV canal is increased by ~9-fold during atrial contraction. However, the magnitude of flow reversal or regurgitation-induced WSS during ventricular contraction decreases by ~33% in comparison with the earlier stages as the AV canal is developing into a valvular structure. (d–i) The ventricle has increased in size when compared to the atrium and absolute values of velocity have increased by 33%, A, atrium; V, ventricle. |
|
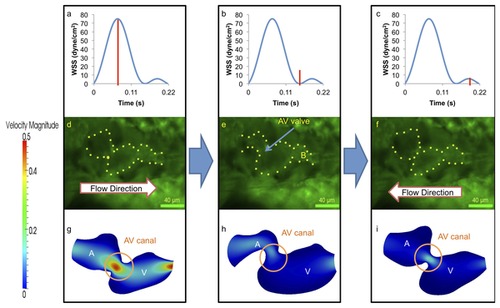
At 110-120 hpf, the morphologic AV valve is distinct and left ventricle is enlarging. (a–c) WSS across AV valve in response to atrium contraction is significantly higher than that of ventricular contraction. (d–i) Complete formation of the AV valve and bulbus arteriosus was observed at this stage, and amount of flow reversal reduced. The ventricle has become larger than the atrium in size, and the flow reversal through the AV canal during ventricular contraction is reduced due to small size of AV canal. A, atrium; V, ventricle; B, bulbus arteriosus. |