- Title
-
The PCP protein Vangl2 regulates migration of hindbrain motor neurons by acting in floor plate cells, and independently of cilia function
- Authors
- Sittaramane, V., Pan, X., Glasco, D.M., Huang, P., Gurung, S., Bock, A., Li, S., Wang, H., Kawakami, K., Matise, M.P., and Chandrasekhar, A.
- Source
- Full text @ Dev. Biol.
|
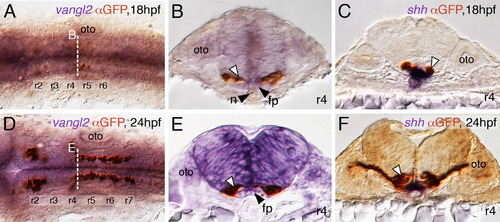
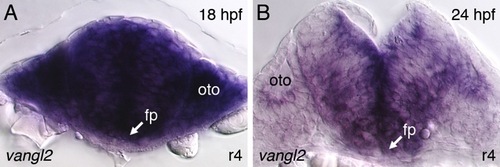
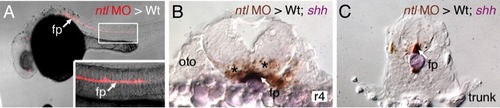
FBM neurons contact vangl2-expressing floor plate cells in rhombomere 4. (A,D) Dorsal views of Tg(islet1:GFP) hindbrains processed for vangl2 in situs (purple) and anti-GFP immunostaining to label FBM neurons (brown). The level of the cross-sections is indicated by the broken lines. (B,E) vangl2 is expressed broadly in the endoderm, mesoderm and within the neural tube, including the floor plate (fp, black arrowhead). Some FBM neurons (white arrowhead) are immediately adjacent to the floor plate cells. (C,F) FBM neuron cell bodies and processes (white arrowhead) contact floor plate cells marked by shh expression (purple). n, notochord; oto, otocyst. EXPRESSION / LABELING:
|
|
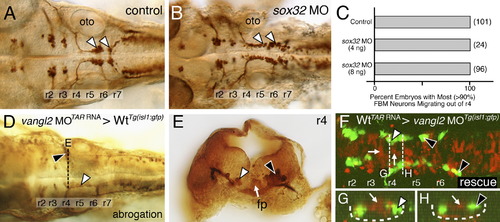
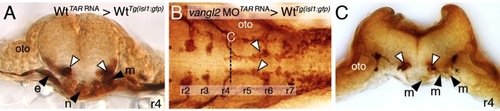
Ventral neural tube expression of vangl2, but not the endoderm, is required for FBM neuron migration. (A,B) Dorsal views of 36 hpf Tg(isl1:GFP) hindbrains stained for FBM neurons (arrowheads, GFP antibody). In sox32 morphant embryos, which lack endoderm, FBM neurons largely migrate normally out of r4, as in wildtype embryos. (C) Quantification of the FBM neuron migration phenotype in sox32 morphant embryos. Number of embryos in parentheses. (D–H) Dorsal views of 36 hpf Tg(isl1:GFP) host hindbrains (D,F), with the level of r4 cross-sections (E,G,H) indicated by broken lines. In D and E, motor neurons and donor-derived cells are both labeled brown due to streptavidin-conjugated alkaline phosphatase reactivity used to detect anti-GFP antibody binding (motor neurons) and biotinylated rhodamine dextran (vangl2 MO donor cells). In F–H, the endogenous fluorescence from GFP and rhodamine were used to identify the motor neurons and donor-derived cells, respectively. Inadvertant targeting of vangl2 morphant cells to the ventral neural tube (white arrow, E) in a wildtype host (D) results in failure of host FBM neurons to migrate (black arrowhead in D,E) on one side, with normal migration on the other (white arrowheads). Conversely, inadvertant targeting of wildtype cells (rhodamine dextran tracer, red) to the ventral neural tube (white arrows, F–H), including the floor plate (not visible due to very weak fluorescence), in a vangl2 morphant host (F) rescues the migration defect of host FBM neurons (black arrowheads, F–H). fp, floor plate; oto, otic vesicle. |
|
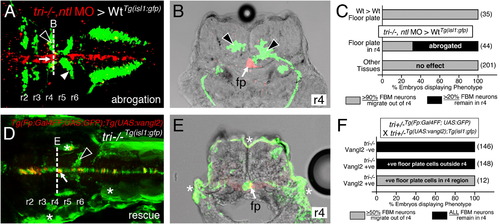
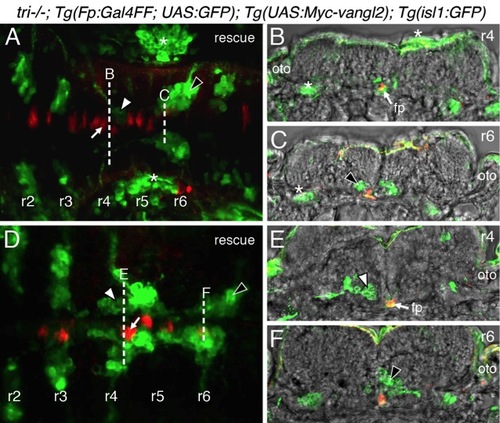
Vangl2 functions in floor plate cells in r4 to regulate FBM neuron migration. Panels A,D show dorsal views of 36 hpf (A) and 48 hpf (D) Tg(isl1:GFP) host hindbrains, and panels B,E show cross-sections at the levels indicated by the broken lines. Vangl2 mutants are indicated as tri/ throughout. (A,B) Presence of vangl2 mutant donor cells in the floor plate (fp) in r4 (arrows) of a wildtype host can abrogate (partially block) the migration of some host FBM neurons (black arrowheads) out of r4. A large number of host neurons (white arrowhead) migrate caudally. (C) Quantification of vangl2 mutant>Wt transplantation data. Number of embryos in parentheses. (D,E) Floor plate-specific expression of vangl2 (arrows) in a vangl2 mutant completely rescues the migration of host FBM neurons (arrowhead). Gal4+ve floor plate cells are green, Myc-Vangl2+ve cells are red or yellow. Asterisks indicate Gal4FF-induced GFP expression in otic vesicle, trunk muscles, and skin. Many GFP-expressing cells are Myc-Vangl2ve (and vice versa) probably due to independent and variable silencing of the UAS:GFP and UAS:Myc-vangl2 transgenes integrated at different sites. (F) Quantification of Gal4-UAS rescue data. Number of embryos in parentheses. fp, floor plate. |
|
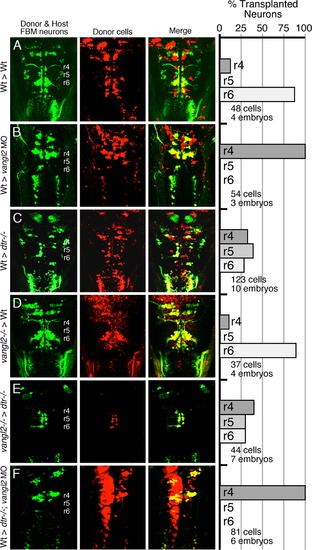
Vangl2 functions outside FBM neurons to regulate their migration. (AF) Dorsal views of the hindbrain in 48 hpf host embryos with anterior to the top. Both the donor and host embryos are Tg(isl1:GFP). Donor derived cells contain the lineage tracer rhodamine dextran (red), hence the donor-derived motor neurons are yellow, while the host motor neurons are green. Quantification of non-migrated FBM neurons in r4 and migrated FBM neurons in r5 and r6 is shown in the histograms. Different transplant conditions are indicated on the far left, and written as Donor>Host. Vangl2-/- refers to trilobite mutants. Wildtype, donor-derived motor neurons (yellow cells) in r6 of vangl2 morphant (B) and dtr-/-; vangl2 morphant (F) host embryos are not scored as FBM neurons because they do not have anteriorly directed axons exiting in r4, and are likely glossopharyngeal motor neurons. (A–D) depict control experiments. (E) Many vangl2 mutant FBM neurons migrate out of r4 in a vangl2+/+ environment, even in the absence of host wildtype FBM neurons. (F) Conversely, wildtype FBM neurons never migrate out of r4 in a vangl2-deficient environment, even in the absence of host mutant FBM neurons (compare to B). |
|
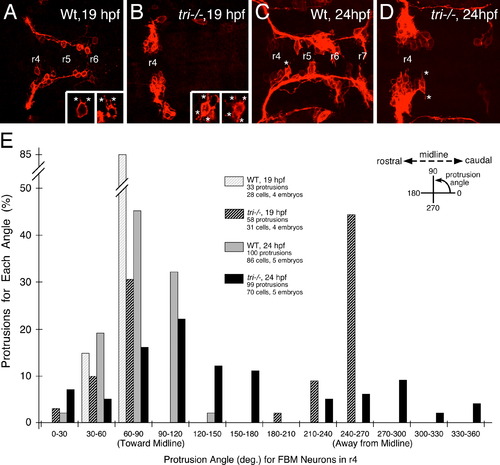
Loss of orientated FBM neuron protrusions in vangl2 mutants. (AD) Dorsal views of Tg(zCREST:mRFP) hindbrains processed for RFP immunostaining. In wildtype embryos (A,C and insets), protrusions (asterisks) generated by FBM neurons in r4 are oriented toward the midline. In vangl2 mutant (tri/) embryos (B,D and insets), the protrusions of FBM neurons in r4 are oriented in various directions, with a large number directed away from the midline. (E) Quantification of the orientation of protrusions of FBM neurons in r4 of wildtype and vangl2 mutant embryos. Whereas the orientation angles for all wildtype protrusions at 19 and 24 hpf are limited to the quadrants directed toward the midline, a large number of protrusions of mutant neurons are directed 180 degrees away from the midline, especially at 19 hpf (significant difference from wildtype at p<0.001; Watson–Williams F-test). EXPRESSION / LABELING:
PHENOTYPE:
|
|
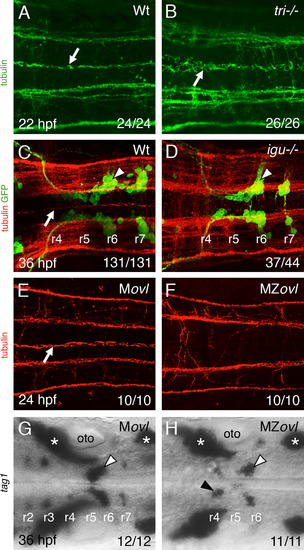
Floor plate cilia are largely dispensable for FBM neuron migration. (A–H) Dorsal views of hindbrains with anterior to the left. The ratio of the number of embryos exhibiting a phenotype to the total number of embryos examined is indicated in each panel. (A,B) Anti-tubulin immunostaining (green). In a wildtype hindbrain (A), floor plate cilia (arrow) are uniformly orientated along the anterior-posterior axis, whereas in a vangl2 mutant (tri/) mutant (B), the floor plate cilia (arrow) are disorganized and misorientated. (C,D) Tg(isl1:GFP) embryos processed for anti-tubulin (red) and anti-GFP (green) immunostaining. In a wildtype sibling (C), floor plate cilia (arrow) are uniformly orientated, and FBM neurons (arrowhead) migrate out of r4. In an iguana/ embryo (D), although floor plate cilia are absent, FBM neurons (arrowhead) migrate normally out of r4. (E,F) While floor plate cilia (white arrow) are present and uniformly orientated in the Movl hindbrain (E), they are absent in the MZovl hindbrain (F). Prominent longitudinal axon tracts appear unaffected in MZovl embryos. (G,H) Tag1 in situs to label branchiomotor and sensory neurons. FBM neurons (white arrowheads) migrate out of r4 in both Movl and MZovl embryos, although a small number of neurons (black arrowhead) consistently remained in r4 in MZovl embryos. Asterisks indicate the location of sensory ganglia, which develop normally in both genotypes. EXPRESSION / LABELING:
PHENOTYPE:
|
|
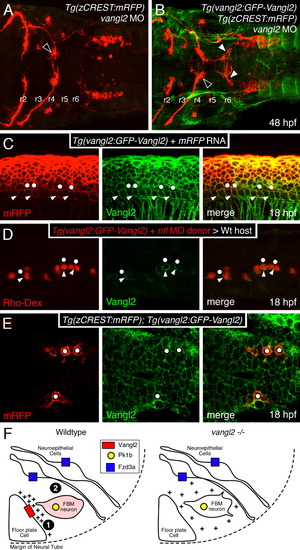
Vangl2 is enriched in basolateral membranes of floor plate cells. Anterior is to the left in panels A–E. (A,B) Dorsal views of hindbrains processed for RFP and GFP immunostaining. In a control vangl2 morphant embryo (A), FBM neurons fail to migrate out of r4 (black arrowhead). In a Tg(vangl2:GFP-Vangl2) vangl2 morphant (B), many FBM neurons migrate out of r4 (white arrowheads). (C) Lateral view of an embryo processed for immunostaining to detect mRFP and GFP-Vangl2. Whereas mRFP is present on basolateral (arrowheads) and apical (dots) surfaces of floor plate cells, the apical surfaces are mostly devoid of GFP-Vangl2. (D) Lateral view of a host embryo containing donor-derived cells targeted to the floor plate, and processed for Rhodamine and GFP immunostaining. Donor-derived floor plate cells (Rho-Dex) exhibit basolateral enrichment of GFP-Vangl2 (arrowheads) with weaker signal at the apical surface (dots). (E) Dorsal view of the hindbrain showing uniform distribution of GFP-Vangl2 on the surface (mRFP) of FBM neurons (dots). (F) Model for regulation of FBM neuron migration by floor plate-derived Vangl2. In the wildtype rhombomere 4 hemi cross-section, Vangl2 on basolateral membranes of floor plate cells sequester a floor plate-derived orientating cue (+). Orientated protrusions on FBM neurons and associated signaling make them competent (primed; denoted by pink shading) to respond to other environmental cues (Step 1). These primed FBM neurons may respond to cell adhesive and/or cell repulsive cues in a Pk1b- and Fzd3a-dependent manner (Step 2), get excluded from the neuroepithelium in r4, and migrate caudally, as proposed previously (Wada et al., 2006). In rhombomere 4 of vangl2 mutants, the floor plate-derived cue does not get sequestered at the basolateral membranes of floor plate cells, and the FBM neurons do not become competent (Step 1 fails). The neurons are unable to respond to adhesive/repulsive cues on neuroepithelial cells, resulting in their integration into the neuroepithelium in r4 and consequent failure to migrate caudally. The depiction of Vangl2 and Fzd3a on floor plate and neuroepithelial cells, respectively, indicates their functional requirement for neuronal migration, and not their expression pattern, which is ubiquitous in the neural tube. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Vangl2 is expressed broadly in the hindbrain and surrounding tissues during the period of FBM neuron migration. |
|
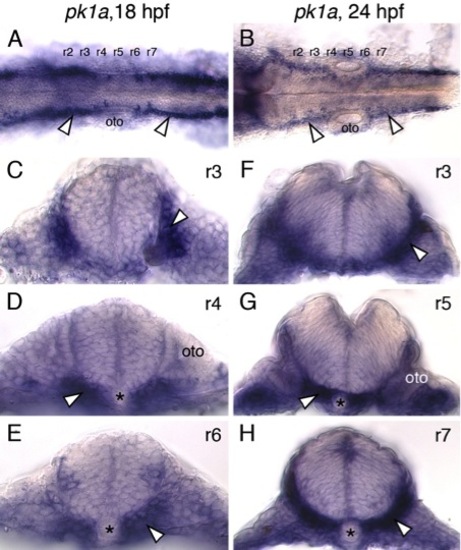
pk1a is expressed primarily outside the neural tube in the paraxial mesoderm and endoderm. (A,B) Dorsal views of zebrafish hindbrains at 18 and 24 hpf processed for pk1a in situs are suggestive of pk1a expression in the lateral regions of the neural tube (arrowheads). However, cross-sections at various levels through the hindbrain (C-H) reveal that pk1a is expressed primarily in the paraxial mesoderm and endoderm (arrowheads). Asterisks and oto indicate notochord and otic vesicle, respectively. |
|
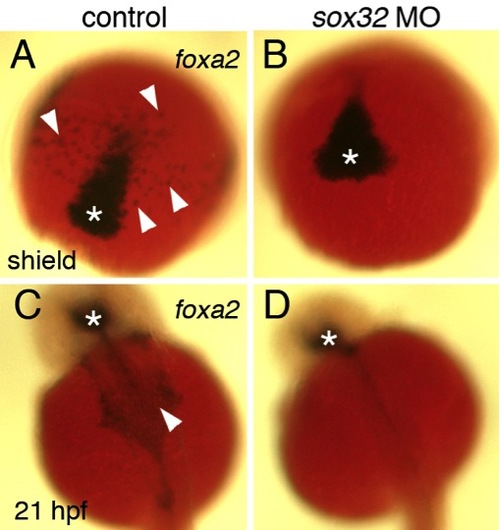
Absence of cranial endoderm in sox32 morphants. |
|
Mesodermal vangl2 expression is not required for FBM neuron migration. |
|
Targeting of transplanted donor cells to the floor plate of host embryos. |
|
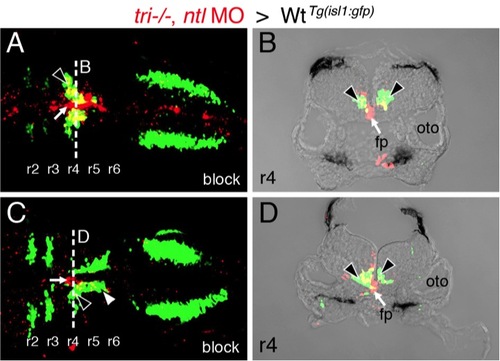
Presence of vangl2 mutant floor plate cells in r4 can block the migration of wildtype FBM neurons. |
|
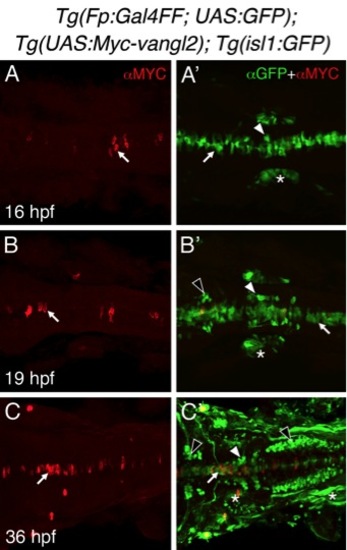
Tg(Fp:Gal4FF), and Tg(UAS:Myc-vangl2) transgenes induce mosaic expression of Myc-Vangl2 in the floor plate. |
|
Expression of vangl2 transgene in floor plate cells can rescue FBM neuron migration in vangl2 (tri-/-) mutants. |
|
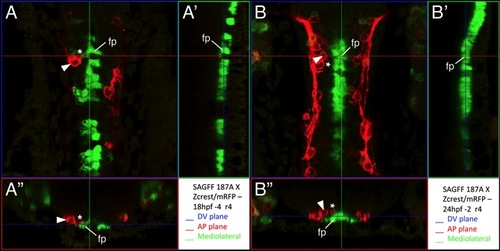
Some FBM neuron protrusions contact floor plate cells |
|
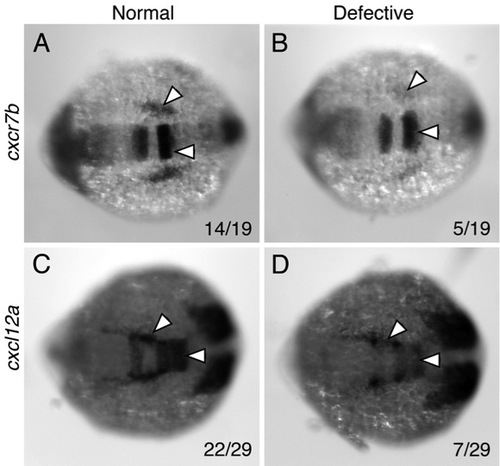
Aberrant chemokine gene expression in putative detour mutants |
Reprinted from Developmental Biology, 382(2), Sittaramane, V., Pan, X., Glasco, D.M., Huang, P., Gurung, S., Bock, A., Li, S., Wang, H., Kawakami, K., Matise, M.P., and Chandrasekhar, A., The PCP protein Vangl2 regulates migration of hindbrain motor neurons by acting in floor plate cells, and independently of cilia function, 400-412, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.