- Title
-
Dorsomorphin promotes survival and germline competence of zebrafish spermatogonial stem cells in culture
- Authors
- Wong, T.T., and Collodi, P.
- Source
- Full text @ PLoS One
|
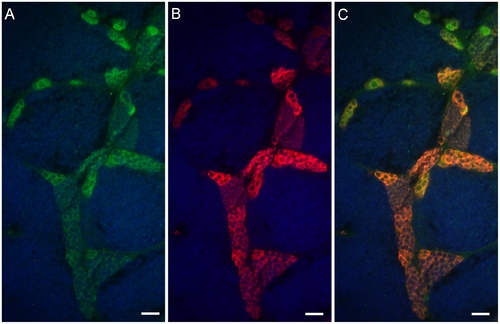
Neo expression overlaps with Vasa in the testicular germ cells of Tg(piwil1:neo) fish. Photomicrographs showing that (A) Neo (green) and (B) Vasa (red) are expressed in the same testicular germ cells including the spermatogonia of Tg(piwil1:neo); (C) merged photo of A and B. The section was also stained with DAPI (Blue). Scale bar = 20 μm. |
|
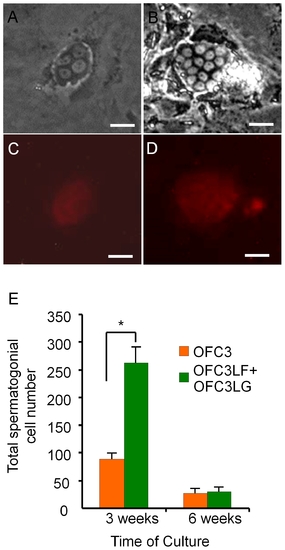
Feeder cells expressing zebrafish Lif, Fgf2 and Gdnf promote spermatogonial cell proliferation for 3 weeks in culture. Photomicrographs showing G418-selected spermatogonia that were initiated from Tg(piwil1:neo);Tg(piwil1:DsRed) zebrafish showing (A) 4-cell and (B) 15-cell colonies; DsRed expression in the (C) 4-cell and (D) 15-cell colonies. (E) OFC3LF and OFC3LG significantly enhanced spermatogonial cell proliferation in cultures maintained for 3 weeks while the mitogenic effect was lost after 6 weeks. * indicates a significant difference by Student t-tests. Scale bar = 20 μm. |
|
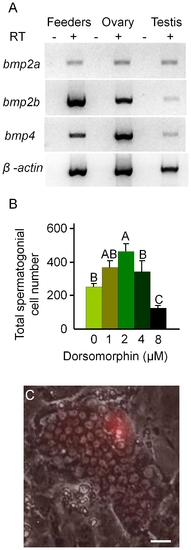
Dorsomorphin promotes the growth of spermatogonia in 3 week cultures. (A) RT-PCR results showing that feeder cells (OFC3LF+OFC3LG) express bmp2a, bmp2b and bmp4. (B) Addition of dorsomorphin significantly (p<0.001) enhanced spermatogonial cell proliferation in 3 week cultures. (C) Merged bright field and UV photomicrographs showing a colony containing approximately 65 DsRed-expressing spermatogonia. Data points not sharing a letter (A, B, C) are significantly different by Bonferroni–Dunn tests. Scale bar = 20 μm. |
|
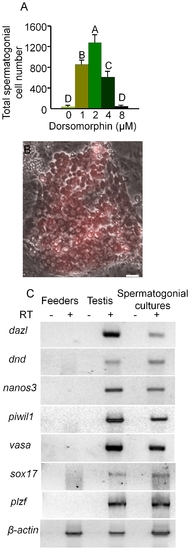
Dorsomorphin increased the growth of spermatogonia in 6 week cultures. (A) Addition of dorsomorphin significantly (p<0.001) increased the growth of spermatogonia maintained in culture for 6 weeks. (B) Merged bright field and UV photomicrographs showing a colony containing more than 100 DsRed-expressing spermatogonia. (C) RT-PCR analysis of RNA isolated from spermatogonia maintained for 6-weeks in culture in the presence of dorsomorphin, whole testis tissue and feeder cells alone showing expression of germ cell specific marker genes, dazl, dnd, nanos3, vasa and piwil1 and spermatogonial markers plzf and sox17. RT: reverse transcription; Data points not sharing a letter (A, B, C, D) are significantly different by Bonferroni–Dunn tests. Scale bar = 20 μm. |
|
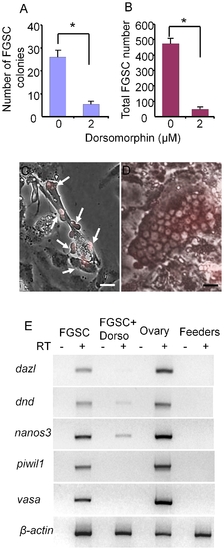
Dorsomorphin inhibits the growth of FGSCs in 3 week cultures. (A) Addition of dorsomorphin significantly inhibits FGSC (A) colony formation and (B) cell proliferation in culture. Photomicrographs showing (C) a 3-week old FGSCs (arrows) cultured in the presence of 2 μM dorsomorphin and (D) a 3-week-old FGSC colony cultured in the absence of dorsomorphin. (E) RT-PCR analysis of RNA isolated from a 3-week FGSC culture grown in the presence or absence of dorsomorphin, whole ovaries and feeder cells alone. Dorsomorphin severely reduced the expression of germ cell specific marker genes, dazl, dnd, nanos3, vasa and piwil1. * indicates a significant difference by Student t-tests. Scale bar = 20 μm. |
|
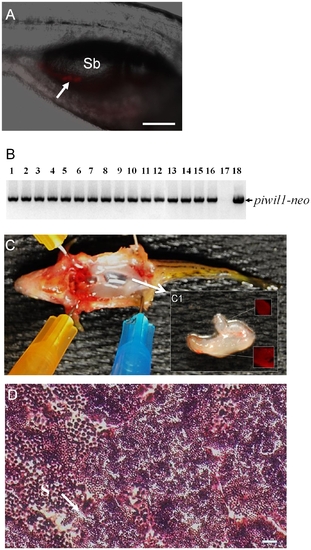
Germline transmission of spermatogonia cultured for 6 weeks in the presence of dorsomorphin. (A) Photomicrograph showing the incorporation of DsRed-positive cultured spermatogonia (arrow) into the gonad of a recipient larva two weeks after transplantation. (B) Results of genomic PCR showing the presence of piwil1-neo sequences that were inherited by all of the F1 individuals (lanes 1 to 16) produced by a germline chimeric father. Negative control: genomic DNA template from a wild-type larva (lane 17); positive control: pPiwil1-neo plasmid DNA template (lane 18). (C) Dissection of a fertile adult male recipient fish showing that the transplanted DsRed-positive spermatogonia have proliferated and directed the formation of a pair of unequal sized testes (arrow) in the body. (C1) Inset shows the gonad under UV light revealing the presence of DsRed-positive cells. (D) Transverse section of testis from a fertile recipient fish showing active spermatogenesis. Sb: swim bladder; S: spermatozoa (arrow). Scale bar = 100 μm for A and 20 μm for D. |