- Title
-
Calnexin is required for zebrafish posterior lateral line development
- Authors
- Hung, I.C., Cherng, B.W., Hsu, W.M., and Lee, S.J.
- Source
- Full text @ Int. J. Dev. Biol.
|
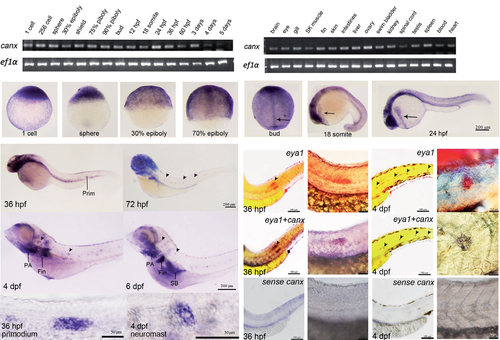
Spatial and temporal expression patterns of calnexin in developing zebrafish embryos. RT-PCR analyses of calnexin were performed using cDNAs from embryos at designated stages (A) and in different adult tissues (B). A 107-bp calnexin fragment was amplified, and a 524-bp ef1α fragment was also amplified as an internal control. (C-U′) Whole-mount in situ hybridization by a calnexin antisense riboprobe was performed in embryos at designated stages. All photographs shown are in lateral view except for F and G which are in dorsal view; (H-U′) anterior to the left, dorsal to the top. Arrows indicate the notochord (G) and hatching gland (H, I). Arrowheads point to selected neuromasts (K-M); Large scale view of primordium (N) and neuromast (O). (P-S) Co-expression assay eya1 and canx. Embryos were fixed at designated times and subjected to WISH against indicated genes. Arrows point to primordia in panels P and R and neuromasts in panels Q and S, respectively. Enlarged images are shown in panels P′-S′. hpf, hours post-fertilization; dpf, days post-fertilization; Prim, primordium; PA, pharyngeal arch; SB, swim bladder. |
|
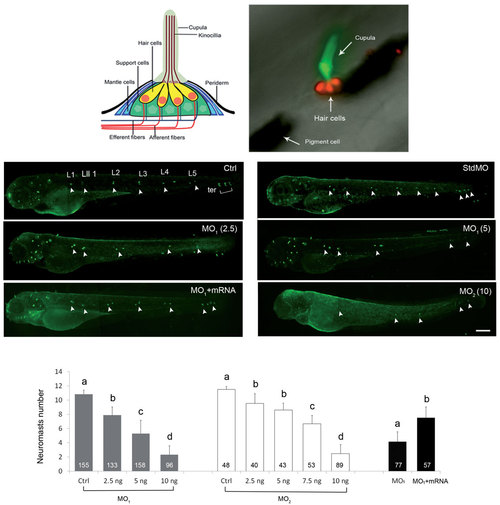
Knockdown of calnexin dose-dependently inhibited the formation of neuromasts. (A) Diagram of a fish neuromast (modified from (Ma and Raible, 2009). (B) A zebrafish neuromast was labeled by WGA 488 (green) and DASPEI (red) to respectively reveal its cupula and hair cells. Two large black patches are pigments. (C-I) Embryos were treated without or with indicated morpholino oligonucleotide (MO; ng/embryo) of a standard control MO (stdMO, 5 ng), calnexin MO1 or calnexin MO2, incubated until 3 days post-fertilization, stained with WGA 488, and observed and photographed under epifluorescence microscopy. calnexin mRNAs (25 pg) was added for MO rescue (G). Photographs are shown in lateral view with the anterior toward the left and posterior to the right. Only left side trunk neuromasts are indicated by white arrowheads. Trunk neuromasts are designated L1~ L5, Lll1, and ter as shown in untreated larvae (C, Ctrl). The vague green fluorescent spots are out-of-focus neuromasts on the opposite side of the trunk. Numbers of left side neuromasts were counted (I). Different letters at the top of each column indicate a significant difference between groups at p < 0.05. Scale bar: 200 am. The number within each column is the sample size. PHENOTYPE:
|
|
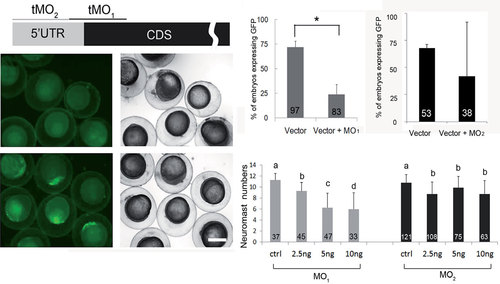
Knockdown of calreticulin causes a reduction in posterior lateral line neuromasts. (A) A partial mRNA map of calreticulin is presented with indicated MO binding sites. The potency of MO1 to reduce calreticulin expression was shown by co-injecting 150 pg of the pCS2+-CRT-GFP-XLT plasmid with (B,C) or without (D,E) calreticulin MO1; then it was cultured, observed under a bright (C,E) or dark field (B,D), and photographed at 10 hpf. The percentage (%) of green fluorescent protein (GFP)-expressing embryos for 5ng calreticulin MO1 or calreticulin MO2 for each treatment is shown as the mean ± standard error in (F,G), respectively. Embryos were treated, observed and shown as described previously. Numbers of neuromasts on the left side of the trunk in all treatments were counted and are shown in (H). Different letters at the top of each column indicate a significant difference between groups at p < 0.05. Scale bar: 200 µm. The number within each column is the sample size. PHENOTYPE:
|
|
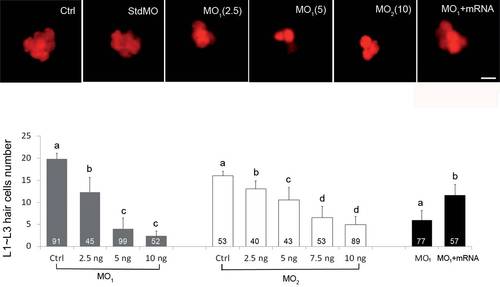
Knockdown of calnexin dose-dependently inhibited neuromast hair-cell formation. Embryos were treated with different dosages of morpholino and calnexin mRNA are stained with DASPEI instead of WGA488 at 72 hpf to reveal neuromast hair cells. Representative photographs of one neuromast are shown in (A). Total hair-cell numbers of the L1~L3 trunk neuromasts in all treatments were counted and are shown in (B). Scale bar: 25 µm. The number within each column is the sample size. PHENOTYPE:
|
|
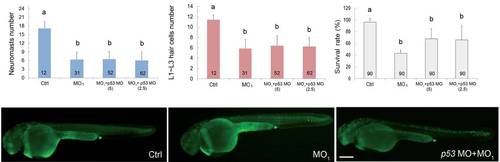
Calnexin morpholino oligonucleotide (MO)-induced lateral line defects are independent of p53. Embryos were treated without (Ctrl) or with 5 ng of the calnexin MO1 (MO) in the presence or absence of 2.5 or 5 ng of p53 MO until 3 day post-fertilization (dpf). Numbers of trunk neuromasts (A) and neuromast hair cells (B), and the survival rate (C) are shown. Treated embryos were also stained with the vital dye, acridine orange, at 36 hpf to observe apoptotic cells and photographed under epifluorescent microscopy. Representative photographs for all treatments are shown in lateral view with the anterior to the left and posterior to the right (D-F). Scale bar: 200 µm. The number within each column represents the sample size. PHENOTYPE:
|
|
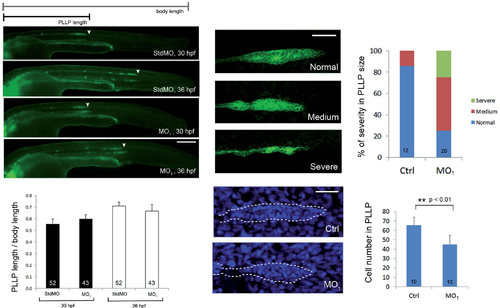
Loss of calnexin reduced primordium size but did not affect migration of the posterior lateral line primordium (PLLP). (A-D) Transgenic claudin b:gfp embryos were treated with 5 ng std MO and with 3.75 ng of the calnexin MO1 and imaged at 30 and 36 hpf. Embryos were observed and photographed under epifluorescence microscopy. Scale bar: 200 µm. (E) Ratios of the migration path of the PLLP to body length at 30 and 36 hpf are shown. (F-K) Transgenic claudin b:gfp embryos were treated without or with MO1, cultured until 32 hpf, stained with DAPI and examined under confocal microscopy for GFP (excitation: 455-490 nm; emission 500-540 nm) and DAPI signal (excitation: 350 nm; emission 470 nm), respectively. Representative confocal photographs of different severity of PLLP defects are shown in (F-H) and the percentages of embryos with different severities are shown in (I). Photographs of control embryos (J) and MO1 morphants (K) stained with DAPI are shown. (L) The average cell numbers in PLLPs are shown. * *Indicates that the value significantly differs from that of control embryos (p < 0.001).Scale bar: 50 +m. Photographs are shown in lateral view with the anterior to the left and posterior to the right. |
|
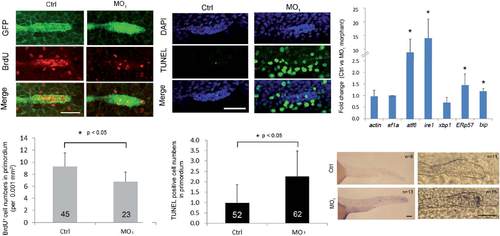
Loss of calnexin inhibited cell proliferation and enhanced apoptosis in the posterior lateral line primordium (PLLP). (A) Transgenic claudin b:gfp embryos were treated without and with 3.75 ng of the calnexin MO1 and collected at 32 hpf. Embryos were labeled with BrdU to reveal proliferating cell and the PLLP was shown by immunostaining against GFP (green). Embryos were examined under confocal microscopy. Photographs are shown in each channel and superimposed images are shown at the bottom. (B) The proliferation rate was estimated by the average number of BrdU-positive cells per 0.001 mm2 of PLLP. (C) Nuclei of treated embryos were stained with DAPI in blue and apoptotic cells were revealed by a TUNEL staining in green. The average numbers of apoptotic cells in the PLLP are shown in (D). (E) Embryos were treated as above and subjected to real-time PCR analysis against designated endoplasmic reticular stress-related genes at 36 hpf. (F) WISH against bip was performed and photographed to show the posterior (left) and PLLP (right) regions. Black dotted lines outline the PLLP region. * The value significantly differs from that of control embryos (p < 0.05). Photographs are shown with the anterior to the left. Scale bar: 50 µm. |