- Title
-
Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin
- Authors
- Glenn, T.D., and Talbot, W.S.
- Source
- Full text @ Development
|
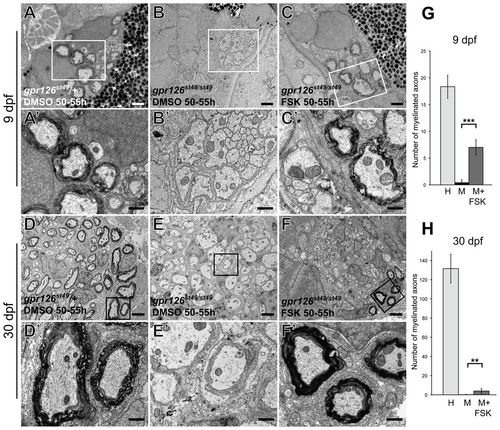
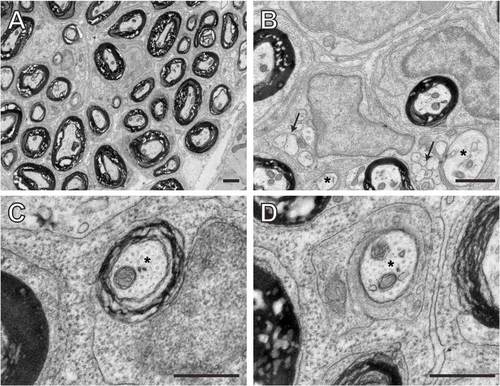
Schwann cell membrane wrapping, compaction, and the maturation of the myelin sheath proceed in gpr126 mutant nerves after transient elevation of cAMP. (A-C2) TEM images of transverse sections through zebrafish larvae at 9 dpf showing the ultrastructure of the PLLn one week following a pulse treatment with either DMSO (A,B) or 50 μM FSK (C). (A) gpr126st49/+ control DMSO-treated fish have normal myelination in the PLLn. (B) The PLLn of gpr126st49/st49 mutant larvae has no myelinated axons at 9 dpf. (C) The PLLn of gpr126st49/st49 mutant larvae that were treated with FSK from 50 to 55 hpf contains some rescued myelinated axons. A2-C2 show higher magnification TEM images of the boxed regions in A-C, respectively. (D-F2) TEM images of transverse sections through juvenile zebrafish at 30 dpf showing the ultrastructure of the PLLn four weeks following a pulse treatment with either DMSO (D,E) or 50 μM FSK (F). (D) gpr126st49/+ control DMSO-treated fish have normal myelination in the PLLn at 30 dpf. (E) The PLLn of gpr126st49/st49 mutant fish has no myelinated axons at 30 dpf. (F) The PLLn of gpr126st49/st49 mutant fish that were treated with FSK from 50 to 55 hpf contains a subset of rescued myelinated axons. D2-F2 show higher magnification TEM images of the boxed regions in D-F, respectively. (G,H) Quantification of the number of myelinated axons present per PLL nerve in heterozygous (H), mutant (M) and FSK-treated mutant (M+FSK) nerves at 9 dpf (G) and 30 dpf (H). Error bars represent s.d. Significance with two-tailed Student’s t-test: ***P<0.001, **P<0.01. Scale bars: 1 μm in A-C; 0.5 μm in A2-C2,D2-F2 2 μm in D-F. PHENOTYPE:
|
|
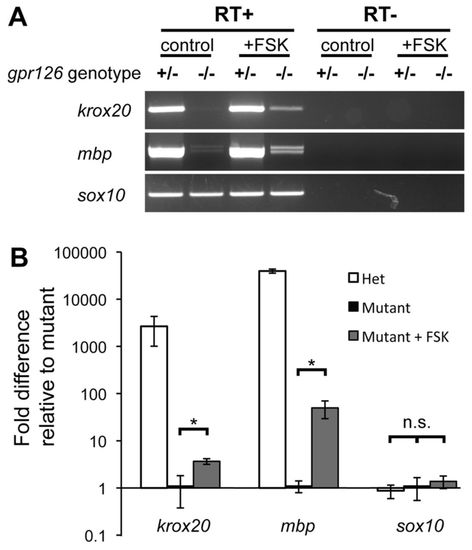
The maintenance of krox20 and mbp expression does not require Gpr126 signaling. (A) RT-PCR from dissected PLL nerves from 3-month-old gpr126st49/+ and gpr126st49/st49 mutant fish showing expression of krox20, mbp and sox10. Fish received either a control pulse of DMSO or 50 μM FSK from 50 to 55 hpf. Mutants that received the FSK treatment show continued expression of krox20 and mbp at 3 months of age, whereas control treated mutants show nearly undetectable levels of both genes. sox10 was expressed at the same level in all samples. (B) qPCR analysis of the mRNA expression levels of krox20, mbp and sox10 in dissected PLL nerves from 6-month-old zebrafish that received treatments as described in A. Fold differences are reported relative to the mutants. Note that the scale is logarithmic. The fold difference between FSK-treated mutants and control mutants for krox20 and mbp was 3.7 and 49.7, respectively. Significance with two-tailed Student’s t-test: *P<0.05; n.s., not significant. Error bars represent s.e.m. |
|
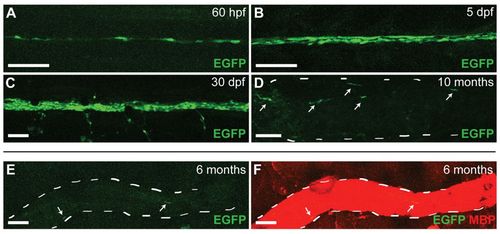
The MSE is highly active in Schwann cells during the initiation of myelination, but activity is downregulated in the mature nerve. (A-D) Lateral views (anterior to the left, dorsal to the top) of wild-type zebrafish imaged live at the indicated stage carrying the MSE:EGFP transgene. EGFP expression is first detected in the PLL nerve at 60 hpf (A), and increases by 5 dpf (B) and 30 dpf (C). At 10 months, only a few Schwann cells express EGFP (D). (E,F) Lateral view of the PLLn from a wild-type fish fixed and stained for MBP expression at 6 months. EGFP expression is nearly undetectable (E), although the nerve is heavily myelinated at this time as indicated by MBP staining (F). The white dashed lines in D-F outline the nerve, and the white arrows mark EGFP-positive Schwann cells. n=at least five transgenic fish at each stage shown. Scale bars: 50 μm. |
|
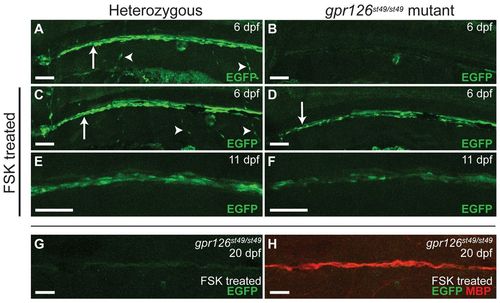
The MSE is not active in Schwann cells of gpr126 mutants, but activity is restored during the initiation of myelination after elevating cAMP. (A-F) Lateral views (anterior to the left, dorsal to the top) of zebrafish larvae imaged live at the indicated stage carrying the MSE:EGFP transgene. Heterozygous fish are in the left column and mutants in the right column. White arrows in A, C and D indicate Schwann cells along the PLLn. Arrowheads in A and C indicate Schwann cells along motor nerves. (A) EGFP-positive Schwann cells are present in gpr126st49/+ fish (n=10/10 transgenic fish). (B) Mutant larvae do not express EGFP in Schwann cells (n=0/6 transgenic fish). (C) Elevating cAMP with a pulse of FSK from 50 to 55 hpf has no effect on MSE:EGFP expression in gpr126st49/+ fish (n=5/5 transgenic fish). (D) Elevating cAMP rescues the expression of MSE:EGFP in Schwann cells along the PLLn in mutant larvae treated with a FSK pulse from 50 to 55 hpf (n=5/5 transgenic fish). (E) Schwann cells along the PLLn continue to express EGFP at 11 dpf in gpr126st49/+ control larvae (n=3/3 transgenic fish). (F) Schwann cells along the PLLn continue to express EGFP at 11 dpf in mutant larvae that were transiently treated with FSK from 50 to 55 hpf (n=7/7 transgenic fish). (G,H) Lateral view of the PLLn from a mutant larva that was treated with FSK from 50 to 55 hpf and then fixed and stained for MBP expression at 20 dpf. EGFP expression is nearly undetectable (G) but MBP expression is strong in rescued Schwann cells (H) (n=10). Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
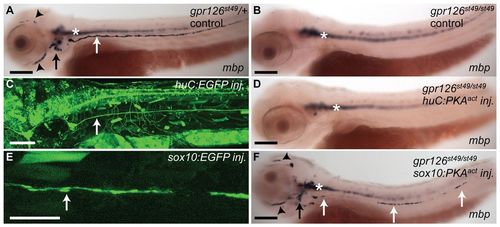
Activated PKA expression in Schwann cells rescues mbp expression in gpr126 mutants. (A,B,D,F) Lateral views of 3 dpf zebrafish embryos showing expression of mbp mRNA. (C,E) Confocal microscope images showing expression of the indicated EGFP reporter constructs. Anterior is to the left and dorsal to the top in all images. (A) mbp is expressed in the PNS of a control gpr126st49/+ embryo, including the PLLn (white arrow), the anterior lateral line (black arrowheads) and cranial ganglia (black arrow) (n=20). Expression is also observed in the CNS (white asterisk). (B) mbp is expressed in the CNS (white asterisk) but not the PNS in a control gpr126st49/st49 mutant embryo (compare expression in A with that in B) (n=10). (C) Zebrafish embryo (3 dpf) injected with the huC:EGFP reporter construct, which drives expression in the nervous system. The white arrow points to axons of the PLLn. Expression was observed in the PLLn of all embryos analyzed (n=8). (D) mbp expression in a gpr126st49/st49 mutant embryo injected with the huC:PKAact transgene. Expression is identical to that seen in control mutant embryos (compare D with B). No mbp expression was observed in the PNS (n=70). (E) 2 dpf zebrafish embryo injected with the sox10:EGFP reporter construct. White arrow points to Schwann cells along the PLLn. Expression in Schwann cells was observed in 13% (n=3/24) of injected embryos. (F) mbp expression in a gpr126st49/st49 embryo injected with the sox10:PKAact transgene. Rescued mbp expression is observed in clones of Schwann cells along the PLLn (white arrows), the anterior lateral line (black arrowheads) and cranial ganglia (black arrow). mbp expression was observed in the PNS in 15% (n=8/52) of injected embryos. Scale bars: 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
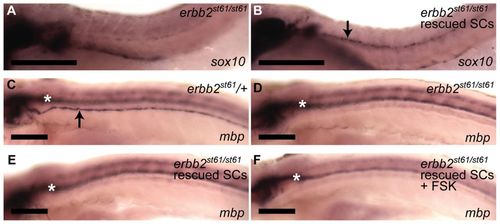
cAMP does not rescue myelination in erbb2 mutants. (A,B) Lateral views of zebrafish embryos at 2 dpf. Anterior is to the left and dorsal to the top in all panels. Schwann cells (marked by sox10, arrow in B) are not present along the PLLn in erbb2st61/st61 mutants (A, n=15), but migration is rescued upon injection of erbb2 mRNA (B, n=38). (C-F) Lateral views of zebrafish larvae at 5 dpf showing expression of mbp in (C) control erbb2st61/+ (arrow indicates PLLn), (D) erbb2st61/st61 mutant, (E) erbb2st61/st61 mutant with rescued Schwann cell migration, and (F) erbb2st61/st61 mutant with rescued Schwann cell migration that was also treated with a FSK pulse from 50 to 55 hpf. mbp expression was not observed in the PLLn of erbb2st61/st61 mutants, even when Schwann cell migration was rescued with erbb2 mRNA injection and cAMP was elevated by FSK treatment. White asterisks mark mbp expression in the CNS. The number of fish analyzed in C-F is: C, n=60; D, n=8; E, n=10; F, n=12. Scale bars: 100 μm. |
|
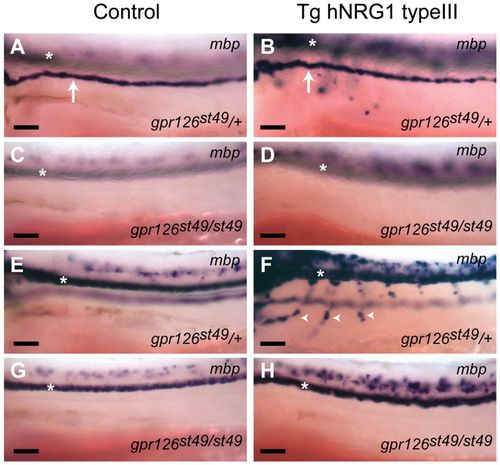
Overexpression of NRG1 Type III does not rescue myelination in peripheral nerves of gpr126 mutants. (A-H) Lateral views (anterior to the left, dorsal to the top) of mbp expression in control (A,C,E,G) or hNRG1 type III transgenic (B,D,F,H) embryos at 3 dpf. The white arrows in A and B mark mbp-expressing Schwann cells of the PLLn, and the white arrowheads in F mark Schwann cells along motor nerves. Control heterozygous embryos at 3 dpf express mbp in the PLLn (A) but not motor nerves (E) (n=35 fish), but transgenic embryos overexpressing hNRG1 type III show precocious mbp expression in motor nerves (F), in addition to expression in the PLLn (B) (n=89 fish). No expression in the PLLn or motor nerves was observed in gpr126st49/st49 mutant embryos in either control (C,G; n=6 fish) or hNRG1 type III transgenic fish (D,H; n=29 fish), although mbp staining in the spinal cord was evident. White asterisks mark CNS expression in all panels. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Schwann cells at different developmental stages in the posterior lateral line nerve at 50 dpf. (A-D) TEM images of transverse sections through adult wild-type zebrafish at 50 dpf showing the ultrastructure of the PLL nerve. (A) Low magnification view of the PLLn showing many myelinated axons. (B-D) Higher magnification views of the PLLn show Schwann cells at multiple stages of development. The arrows in B mark groups of unsorted axons that are ensheathed by immature Schwann cells, and the asterisks mark sorted axons. Multiple myelinated axons are also visible in the same field. (C) A myelinating Schwann cell has elaborated several wraps around an axon (marked with asterisk) although the level of myelination is similar to that observed at earlier stages (compare with Fig. 1). (D) A Schwann cell has established a 1:1 relationship with an axon (marked with asterisk), but has not yet initiated myelination. Scale bars: 1 μM in A,B; 0.5 μM in C,D. |
|
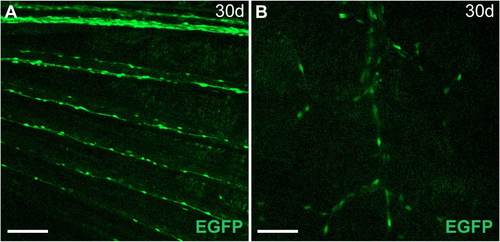
Peripheral nerves that initiate myelination later in development exhibit MSE activity at the appropriate stages (A) Schwann cells along nerves in the pectoral fin express MSE:EGFP at 30 dpf. (B) Schwann cells along axons innervating lateral line stitches, which do not form until the juvenile stage, express EGFP at 30 dpf. Anterior is to the left, and dorsal to the top in both images. Scale bars: 100 μM in A; 50 μM in B. |