- Title
-
Dopamine from the Brain Promotes Spinal Motor Neuron Generation during Development and Adult Regeneration
- Authors
- Reimer, M.M., Norris, A., Ohnmacht, J., Patani, R., Zhong, Z., Dias, T.B., Kuscha, V., Scott, A.L., Chen, Y.C., Rozov, S., Frazer, S.L., Wyatt, C., Higashijima, S., Patton, E.E., Panula, P., Chandran, S., Becker, T., and Becker, C.G.
- Source
- Full text @ Dev. Cell
|
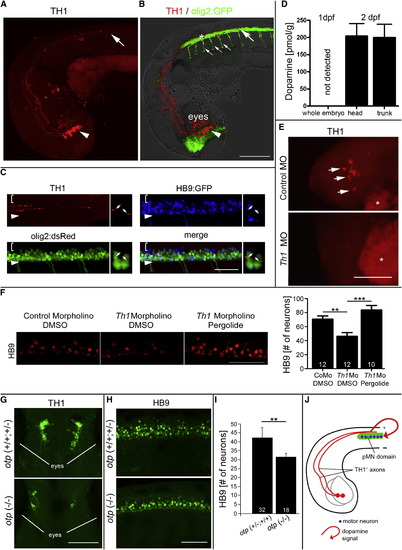
Dopamine from the Brain Promotes Motor Neuron Development (A and B) In a lateral view of (A) TH1 immunohistochemistry only and (B) combined with olig2:GFP fluorescence and light microscopy, diencephalic TH1+ neurons (arrowheads; 33 hpf) and axonal growth cones of their spinal projections (large arrows) are labeled as they grow in close proximity to olig2:GFP+ cells in the ventral spinal cord. Small arrows in (B) indicate motor axons. (C) TH1 labeling reveals close contact of axons (small arrows in coronal views) with pMN progenitors (olig2:dsRed+/HB9:GFP-). Brackets indicate dorsal spinal cord; arrowheads indicate ventral border of spinal cord. (D) Consistent with TH1+ axon growth, HPLC detects dopamine in 48 hpf embryos but not in 24 hpf embryos. (E and F) Injection of a morpholino to th1 leads to (E) loss of TH1 immunoreactivity (indicated by arrows and lateral views of heads; asterisks indicate autofluorescence of the yolk sac) and (F) a significant reduction in the number of HB9+ motor neurons (lateral spinal cord views; 33 hpf), which is fully rescued by pergolide application (drug exposure 24–33 hpf; one-way ANOVA, p = 0.0001, with Bonferroni’s multiple comparison, p < 0.001, p < 0.0001). (G–I) In the otp mutant, fewer dopaminergic neurons are present in the brain, as indicated (G) in dorsal views of heads by TH1 immunohistochemistry, and motor neuron numbers in the spinal cord are reduced, as indicated in (H) lateral views of HB9 immunohistochemistry and (I) quantification thereof. p < 0.01. (J) Model of the spatial relationship of descending dopaminergic axons with the spinal pMN domain. Error bars represent SEM. Scale bars, 200 μm (A and B), 50 μm (C, F, and H), and 100 μm (E and G). See also Figure S1. |
|
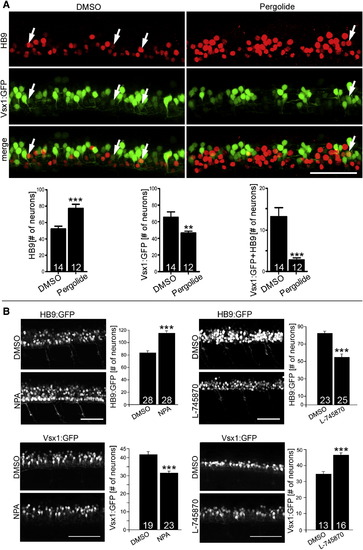
Dopamine Signaling Inversely Affects Motor Neuron and V2 Interneuron Numbers Lateral trunk views at 33 hpf are shown (drug exposure: 24–33 hpf). (A) Treatment with the dopamine agonist pergolide leads to an increase of HB9 immunoreactive motor neurons and a reduction of transgenically labeled presumptive V2 interneurons (vsx1:GFP) in the same embryos. A small subpopulation of double-labeled cells is also strongly reduced (Mann-Whitney U test; p < 0.0001, p < 0.001). (B) NPA, another dopamine agonist, increases the number of HB9:GFP+ motor neurons and decreases the number of vsx1:GFP+ cells (left column). The D4 antagonist L-745870 has the inverse effect on numbers of these cell types (right column). Error bars represent SEM. Scale bars, 50 μm. See also Figure S2. EXPRESSION / LABELING:
|
|
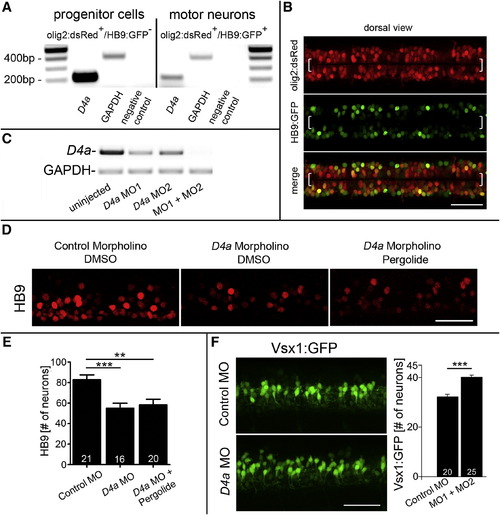
The D4a Receptor Is Necessary to Mediate the Action of Dopamine on Neurogenesis (A) PCR analysis on fluorescence-activated cell-sorted cells from olig2:dsRed/HB9:GFP double-transgenic embryos shows that d4a receptor mRNA is enriched in pMN progenitors, compared to motor neurons. (B) In a dorsal view of double-transgenic embryos, olig2:dsRed+/HB9:GFP- pMN progenitors (brackets) and double-labeled motor neurons are visible. (C) d4a morpholino knockdown is efficient, as shown by PCR. (D) d4a knockdown (MO1) reduces the number of HB9+ motor neurons, which cannot be rescued by pergolide (exposure 24–33 hpf). (E) Quantification of motor neurons in (D) (ANOVA, p = 0.0003 with Bonferroni’s posttest, p < 0.01, p < 0.0001). (F) d4a knockdown increases the number of vsx1:GFP+ interneurons (p < 0.0001). Error bars represent SEM. Scale bars, 50 μm (B, D, and F). See also Figure S3 and Table S1. |
|
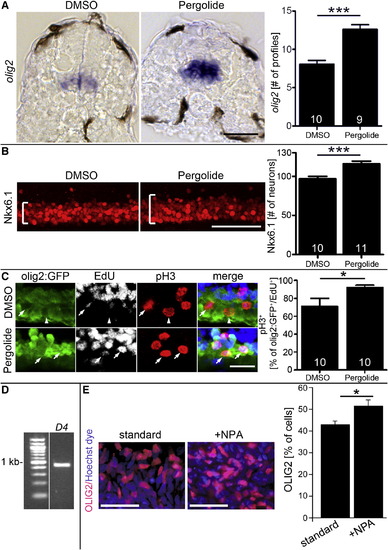
Dopamine Agonists Affect Ventral Progenitor Cells (A) The olig2 mRNA expressing pMN progenitors are increased in number and labeling intensity after pergolide treatment (24–33 hpf; cross-sections; p < 0.0001). (B) Nkx6.1 labeling is expanded (brackets) after pergolide treatment (24–33 hpf; lateral views; p < 0.0001). (C) A higher proportion of pMN progenitors in M phase (olig2:GFP+/pH3+) is labeled with EdU 2 hr after exposure, indicating shortened G2 phase, after pergolide treatment (24–33 hpf; lateral views; p < 0.05; arrows indicate triple-labeled cells, and arrowhead indicates a cell that is olig2:GFP+/pH3+/EdU-). (D and E) In human embryonic stem cell cultures, driven toward motor neuron differentiation, PCR indicates expression of the d4 receptor at day 16 (D) and the proportion of OLIG2+ cells is significantly (p = 0.0109) increased by NPA at day 16 (E). Error bars represent SEM. Scale bars, 50 μm (A, B, and E) and 20 μm (C). See also Figure S4. EXPRESSION / LABELING:
|
|
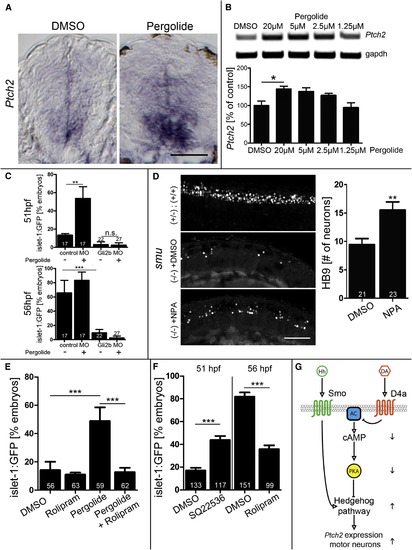
Dopamine Signaling Stimulates the Hedgehog Pathway by Attenuating cAMP/PKA Activity (A) Pergolide increases patched2 (ptch2) expression in in situ hybridization on spinal cross-sections, retaining the ventrodorsal expression gradient (exposure 24–33 hpf). (B) PCR indicates a dose-dependent increase in patched2 expression after pergolide exposure (Kruskal-Wallis, p = 0.0102 with Dunn’s multiple comparison, p < 0.05). (C) Gli2b morpholino inhibits development of islet1:GFP+ motor neurons. This is not rescued by pergolide (56 hpf), capable of promoting motor neuron development in siblings (51 hpf) (p < 0.01, p < 0.0001). (D) NPA partially rescues impaired development of HB9 immunolabeled motor neurons in the smoothened (smu) mutant at 33 hpf (p < 0.0001; lateral trunk views are shown). (E) The phosphodiesterase inhibitor rolipram abolishes the promoting effect of pergolide on development of islet-1:GFP+ motor neurons. (F) The adenylate cyclase inhibitor SQ22536 mimics the action of pergolide by promoting islet-1:GFP+ motor neuron development, and rolipram inhibits development of islet-1:GFP+ motor neurons (p < 0.0001). (G) Model for dopamine action. By inhibiting adenylate cyclase (AC), dopamine (DA), acting through the D4a receptor, reduces the inhibitory influence of cAMP-dependent PKA on the hedgehog pathway, which is normally activated by hedgehog (Hh) acting through the Smoothened receptor (Smo). Upward and downward arrows indicate positive or negative influences, respectively, of D4a activity. Error bars represent SEM. Scale bars, 50 μm (A and D). |
|
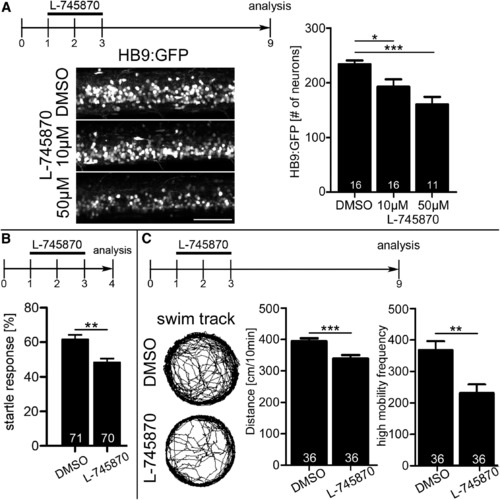
Early Disruption of Dopamine Signaling Has Long-Lasting Consequences for Motor Neuron Numbers and Swimming Behavior (A) Motor neuron numbers (lateral trunk views) are significantly reduced at 9 dpf by exposure to 10 μM L-745870 from 24 to 72 hpf (Kruskal-Wallis p = 0.0102 with Dunn’s multiple comparison, p < 0.05, p < 0.0001). Scale bar, 25 μm. (B and C) Incubation of embryos from 1 to 3 dpf decreases (B) the frequency of a startle response after touch at 4 dpf and (C) the total distance moved and high mobility frequency at 9 dpl (p < 0.01; p < 0.0001). Error bars represent SEM. See also Figure S5. EXPRESSION / LABELING:
|
|
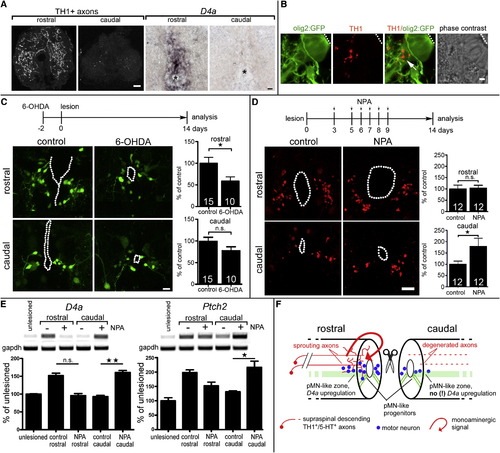
Dopamine Promotes Motor Neuron Regeneration in the Adult Spinal Cord Spinal cross-sections are shown at 14 dpl; central canal is indicated by dots or asterisks. (A) TH1+ axons and d4a mRNA expression are detectable mainly rostral to a spinal lesion site. (B) Higher magnification shows apposition (arrow) of TH1+ axons with radial processes of olig2:GFP+ motor neuron progenitor cells. (C) Ablation of dopaminergic axons with 6-OHDA reduces (p < 0.05) numbers of newly generated HB9:GFP+ motor neurons only rostral to a spinal lesion site. (D) NPA injections significantly (p < 0.05, one sided) increase the number of HB9+ motor neurons only caudal to a spinal lesion site. (E) NPA injections increase d4a (left) and patched2 (right) expression only caudal to the lesion site. Example gels and quantitative densitometric analyses are shown (p < 0.05, p < 0.01; Krukall-Wallis with Dunn’s posttest). (F) Schematic summary of dopamine signaling in relation to motor neuron regeneration at 14 dpl. Error bars represent SEM. Scale bars, 40 μm (A, left), 20 μm (A, right), 5 μm (B), and 15 µm (C and D). See also Figure S6. EXPRESSION / LABELING:
|
|
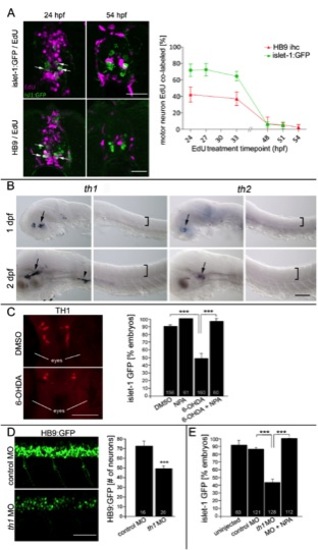
(related to Fig. 1) TH1+ neurons influence the generation of late born motor neurons. A: Exposing embryos to EdU at the given time points leads to different labeling frequencies of islet-1:GFP+ and HB9+ neurons in spinal cross sections at 72 hpf. Over 40% of HB9+ motor neurons and over 70% of islet-1:GFP+ motor neurons are still born after 24 hpf (arrows indicate double labeled cells). Almost all motor neurons are born by 51 hpf. At least 6 larvae were analyzed per time point. B: Th genes are detectably expressed only in the brain. Expression of th1 and th2 mRNA is detectable in the head (arrows) and cranial ganglia (arrowhead), but not in the spinal cord (position indicated by brackets) by in situ hybridization (lateral views) at 1 and 2 days post fertilization (dpf). C: 6-OHDA reduces the number of TH1+ diencephalic neurons (dorsal head views, rostral is up) and attenuates islet-1:GFP+ motor neuron development. This is fully rescued by NPA (exposure 24 to 56 hpf, ANOVA, ***P < 0.0001). D, E: Injection of a morpholino against th1 leads to a significant reduction in the number of HB9:GFP+ motor neurons (D; lateral trunk views; 33 hpf; ***P = 0.001), and attenuation of islet-1:GFP+ motor neuron development (E), which is fully rescued by NPA application (drug exposure 24 to 56 hpf; ANOVA, ***P < 0.0001). Error bars represent SEM. Scale bars: A: 60 μm, B: 100 μm, C: 100μm, D: 50 μm. |
|
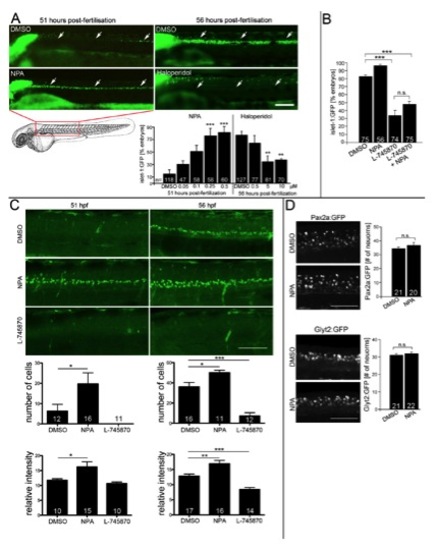
(related to Fig. 2) Dopaminergics influence development of Islet- 1:GFP+ motor neurons, but not Pax2a+ or Glyt2:GFP+ interneurons. A: Lateral views of the trunk region of living islet-1:GFP transgenic animals under a stereo-microscope are shown. The dopamine agonist NPA (drug exposure 24 à 51 hpf; ANOVA, ***P < 0.001 compared to DMSO) significantly increases and the antagonist Haloperidol (drug exposure 24 to 56 hpf; ANOVA, **P < 0.01 compared to DMSO) decreases the percentage of embryos with islet- 1:GFP+ motor neurons (arrows) in a dose dependent fashion. B: The D4 specific antagonist L-745870 attenuates development of islet-1:GFP+ motor neurons, which cannot be overcome by the addition of NPA (56 hpf; ANOVA, ***P < 0.0001). C: Visual scoring of islet-1:GFP embryos is confirmed by cell counts and fluorescence intensity measurements. Lateral views of z-projected confocal image stacks of the mid trunk region are shown. At 51 hpf (left column), cell number (*P < 0.05; Mann-Whitney U-test, one tailed) and fluorescence intensity are significantly increased (*P < 0.05; Mann-Whitney Utest, one tailed) in NPA treated embryos. At 56 hpf (right column), cell number (ANOVA, ***P < 0.0001) and fluorescence intensity (ANOVA, ***P < 0.0001) are significantly lower in L-745870 treated embryos and still increased in NPA treated animals compared to DMSO controls (ANOVA, *P < 0.05; **P < 0.01). Fluorescence intensity of an expression domain in the cerebellum was unchanged by any of the treatments (data not shown). D: Lateral trunk views of embryos at 33 hpf are shown (drug exposure 24 to 33 hpf; genotypes and drugs are indicated). NPA has no effect on the numbers of glyt2:GFP+ or pax2a:GFP+ interneurons. Error bars represent SEM. Scale bars: A: 100 μm; C: 60 μm, D: 50 μm. |
|
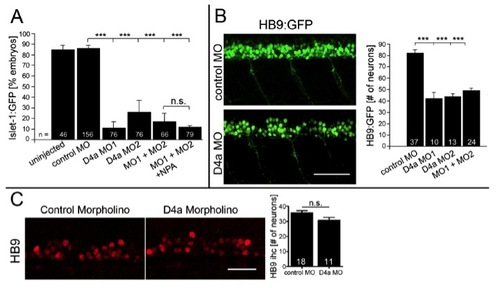
(related to Fig. 3) D4a receptor knock down reduces the number of different motor neuron classes. Lateral views at 33 hpf are shown. A: D4a knock down attenuates islet-1:GFP+ motor neuron development, which is not rescued by NPA (drug exposure 24 to 56 hpf; ANOVA, ***P < 0.0001). B: Numbers of HB9:GFP+ motor neurons are significantly reduced by morpholino treatment (24 to 33 hpf). C: Knock down of d4a had no effect on motor neuron numbers at segment 16-17, when TH1+ axons had only reached trunk segment 7. Error bars represent SEM. Scale bars: 50 μm. |
|
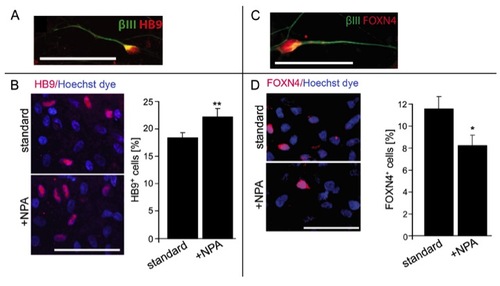
(related to Fig. 4) NPA increases motor neuron numbers and decreases V2 interneuron numbers in human ES cell cultures. A,B: At day 44, HB9+/βIII tubulin+ cells are present (A) and the percentage of HB9+ motor neurons (B) is increased (P = 0.0036) by NPA treatment. C,D: At day 26, FOXN4+/βIII tubulin+ interneurons are present in the cultures (C) and the percentage of FOXN4+ interneurons is decreased by NPA treatment (D; P < 0.05). Error bars represent SEM. Scale bars: 50 μm. |
|
(related to Fig. 6) Targeted ablation of motor neurons impairs escape response. In Tg(HB9:Gal4 x UAS:NTR-mCherry) fish, motor neurons are labeled (top left), which are ablated after Mtz exposure (bottom left, lateral views). The number of HB9+ motor neurons (arrowheads in cross sections, right column) is reduced by 28% (***P < 0.0001), as is the frequency of a startle response elicited by touch (control: 92%, MTZ treated: 35%; ***P < 0.0001). Error bars represent SEM. Scale bars: 50 μm. |
|
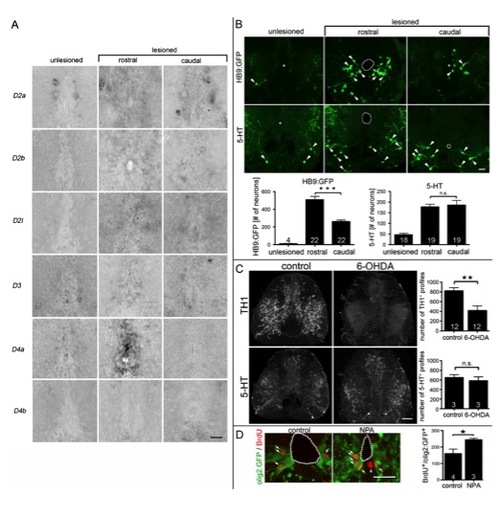
(related to Fig. 7) Dopaminergic control of adult motor neuron regeneration. A: D4a, in contrast to other D2-like receptors, is strongly upregulated in the ependymal zone of the lesioned spinal cord only rostral to the lesion site at 14 days dpl. Spinal cross sections are shown (dorsal up). B: Small intensely labeled HB9:GFP+ motor neurons (arrowheads) are rarely found in unlesioned animals. After a lesion, significantly more (1.9x) HB9:GFP+ motor neurons are generated rostral than caudal to a spinal lesion site (***P < 0.0001). Numbers of 5-HT+ neurons (arrowheads) are strongly increased compared to those in unlesioned animals, but there is no rostrocaudal difference in number (P > 0.05). C: 6-OHDA destroys TH1+ axons, but not serotonergic (5-HT+) axons. Complete cross sections through the spinal cord are shown; dorsal is up. For control and 6-OHDA treatment, respectively, double immuno-fluorescent labeling of the same tissue section is shown. Whereas an intraperitoneal injection of 6-OHDA significantly (**P < 0.01) reduces the number of TH1+ axons, no effect on 5-HT+ axons was detectable (P > 0.05), indicating selectivity of the toxin. D: The number of BrdU+/olig2:GFP+ pMN-like cells (arrows) at the central canal (dotted line) was increased when motor neuron numbers were increased after NPA application (3, 6, 9 dpl) at 14 dpl (*P < 0.05, one-sided test). Error bars represent SEM. Scale bars: A: 15 μm; B: 50 μm; C: 40 μm; D: 50 μm. |
Reprinted from Developmental Cell, 25(5), Reimer, M.M., Norris, A., Ohnmacht, J., Patani, R., Zhong, Z., Dias, T.B., Kuscha, V., Scott, A.L., Chen, Y.C., Rozov, S., Frazer, S.L., Wyatt, C., Higashijima, S., Patton, E.E., Panula, P., Chandran, S., Becker, T., and Becker, C.G., Dopamine from the Brain Promotes Spinal Motor Neuron Generation during Development and Adult Regeneration, 478-491, Copyright (2013) with permission from Elsevier. Full text @ Dev. Cell