- Title
-
A novel chemical screening strategy in zebrafish identifies common pathways in embryogenesis and rhabdomyosarcoma development
- Authors
- Le, X., Pugach, E.K., Hettmer, S., Storer, N.Y., Liu, J., Wills, A.A., Dibiase, A., Chen, E.Y., Ignatius, M.S., Poss, K.D., Wagers, A.J., Langenau, D.M., and Zon, L.I.
- Source
- Full text @ Development
|
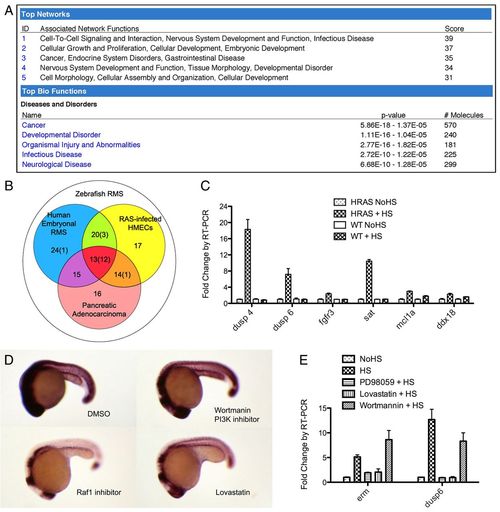
Transient RAS action in Tg(hsp70-HRASG12V) zebrafish embryos resembles pathway activation during tumorigenesis. (A) Ingenuity pathway analysis output for the upregulated gene set identified on comparing Tg(hsp70-HRASG12V) embryos to wild-type zebrafish embryos following heat shock (log fold change >0.5 for top network and >0.7 for top diseases and disorders). (B) RAS signature gene list, defined as commonly upregulated genes in zebrafish RMS (white circle), human embryonal RMS (blue circle), human pancreatic adenocarcinoma (red circle) and RAS-infected HMECs (yellow circle). The total number of genes is shown, with the number tested in the 17-gene list in parentheses [adapted from Langenau et al. (Langenau et al., 2007)]. (C) RT-PCR analysis of six of the RAS signature genes in Tg(hsp70-HRASG12V) and wild-type embryos with and without heat shock. P<0.05 for each gene shown, HRAS+HS compared with other conditions. (D) ISH of erm. Transgenic embryos were incubated with various chemicals from 16 hpf, heat shocked at 18-19 hpf, and fixed at 24 hpf. DMSO, vehicle control. (E) RT-PCR analysis of erm and dusp6 expression levels in response to pathway-specific chemical inhibitors. Error bars indicate s.e.m. HS, heat shock; NoHS, no heat shock. EXPRESSION / LABELING:
|
|
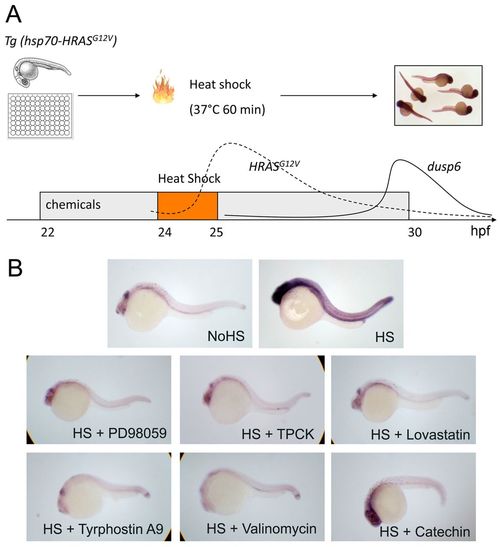
A small-molecule screen in Tg(hsp70-HRASG12V) zebrafish embryos. (A) Scheme of the chemical screen. Heterozygous Tg(hsp70-HRASG12V) embryos were placed in a 48-well plate for chemical treatment starting at 22 hpf and heat shocked from 24-25 hpf in a 37°C waterbath to activate RAS signaling (dashed line). At 30 hpf, embryos were fixed and the dusp6 expression level was evaluated by ISH. The solid line represents the dynamic changes in dusp6 RNA level, as confirmed by RT-PCR; the dashed line represents the predicted activation of RAS based on hsp70 promoter dynamics (Le et al., 2007). (B) ISH of dusp6 on embryos treated with PD98059, TPCK, Lovastatin, Tyrphostin A9, Valinomycin and Catechin. |
|
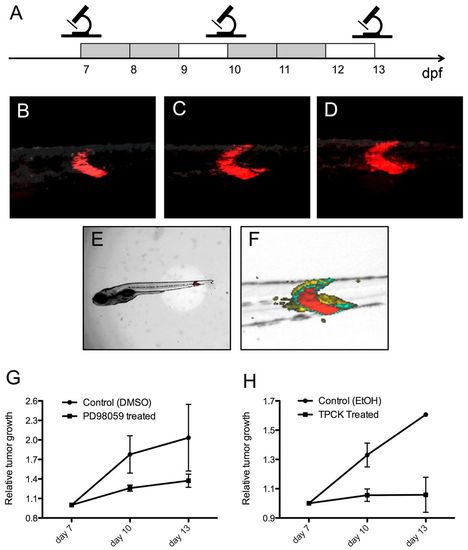
PD98059 and TPCK inhibit tumor progression in rag2-KRASG12D-induced zebrafish ERMS. (A) Scheme of the analysis strategy. Photographs of tumors were taken under standardized conditions at days 7, 10 and 13. Gray boxes indicate days of chemical or control treatment; white boxes represent recovery days. (B-F) Images of a representative tumor-bearing fish receiving vehicle control (DMSO) treatment. (B-D) Images of the tumor area with DsRed fluorescence labeling RAS activation at 7 (B), 10 (C) and 13 (D) dpf. Photographs were taken with an exposure time of 3 seconds and gain of 80%. (E) The overall length of each fish (nose to tail) was also recorded under brightfield illumination at 7, 10 and 13 dpf to ensure the general health of fish. (F) Overlay of B-D, demonstrating the relative growth of the tumor (red, 7 dpf; green, 10 dpf; yellow, 13 dpf). (G,H) Relative tumor growth in fish treated with (G) PD98059 (15.6 μM) or (H) TPCK (0.3 μM) compared with vehicle (DMSO or ethanol) treated siblings (P<0.05, ANOVA, chemical compared with vehicle treatment). Error bars indicate s.e.m. |
|
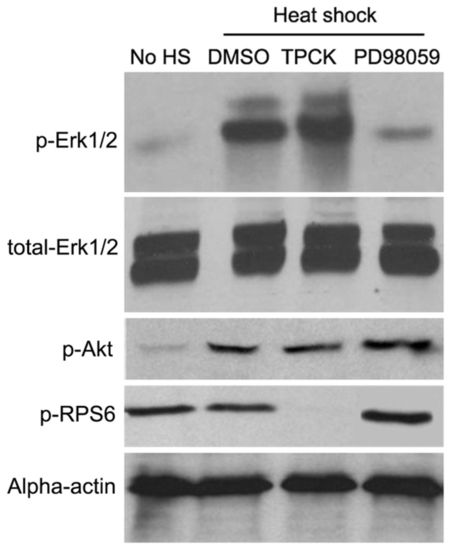
PD98059 and TPCK selectively suppress different downstream RAS signaling pathways in zebrafish embryos. Western blot analysis was performed using Tg(hsp70-HRASG12V) embryos to study the phosphorylation status of Erk1/2 (T202/Y204), Akt (S473) and Rps6 (S240). The embryos were treated with PD98059 (18.7 μM), TPCK (1 μM) or DMSO control from 22 hpf, heat shocked from 24-25 hpf at 37°C and whole embryos were homogenized in 1× SDS sample buffer at 28 hpf. |
|
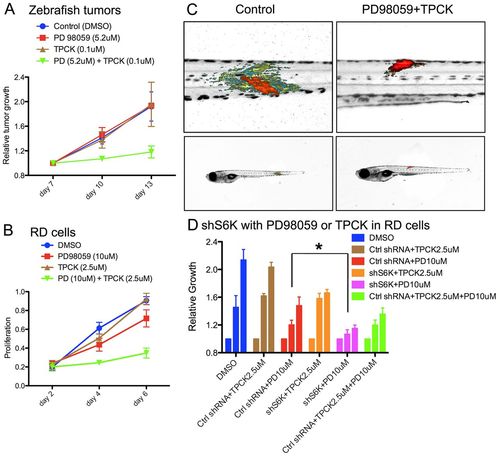
PD98059 and TPCK synergistically suppress tumor progression in zebrafish ERMS and human RD cells. (A) Relative tumor growth in zebrafish ERMS with combination treatment (5.2 μM PD98059 + 0.1 μM TPCK, n=27), PD98059 (5.2 μM, n=13), TPCK (0.1 μM, n=7) and DMSO vehicle [0.28% (v/v), n=12]. P=0.009 (ANOVA, combined treatment compared with other conditoins) at day 10. (B) Human RD cell proliferation measured by MTT assay after vehicle [0.53% (v/v) DMSO], PD98059 (10 μM), TPCK (2.5 μM) or combination treatment (10 μM PD98059 + 2.5 μM TPCK). (C) Representative overlay images of zebrafish with rag2-KRASG12D-induced tumors treated with DMSO vehicle control and a combination of PD98059 and TPCK. Color code as in Fig. 3F. (D) RD cell proliferation measured by MTT assay after cells were treated with DMSO, control (scrambled) shRNA (Ctrl shRNA), S6K1 shRNA (shS6K1), TPCK (2.5 μM) and/or PD98059 (10 μM) as indicated. Three bars of the same color represent (left to right) relative cell growth under a given treatment condition on days 5, 7 and 9. *P<0.05 (ANOVA). Error bars indicate s.e.m. |
|
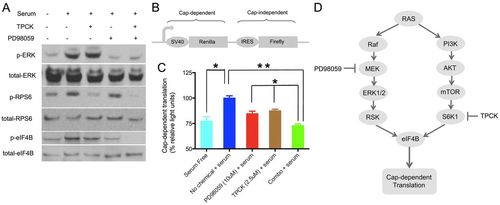
The combination of PD98059 and TPCK suppresses eIF4B phosphorylation and cap-dependent translation initiation. (A) Human RD cells were deprived of serum overnight, treated with vehicle [0.53% (v/v) DMSO], PD98059 (10 μM), TPCK (2.5 μM) or a combination (10 μM PD98059 and 2.5 μM TPCK) for 2 hours, and then stimulated with serum (20%), or continued to be serum-starved, for 30 minutes. The phosphorylation status of ERK1/2 (T202/Y204), RPS6 (S235) and eIF4B (S422) was then analyzed by western blotting. (B) Structure of the bicistronic Renilla/firefly luciferase reporter plasmid used in the translation assay. (C) Cap-dependent translation in chemical-treated RD cells. RD cells were transfected with the reporter plasmid, after serum starvation for 12 hours, and then treated with control, PD98059, TPCK or a combination. Half an hour after chemical exposure, serum (20%) was added to cells to stimulate translation, luciferase activities were measured, and the Renilla/firefly luciferase light unit ratio was calculated. The value of the serum-stimulated sample was set at 100%. The experiment was performed in biological duplicate and technical triplicate. *P<0.05, **P<0.01 (Student′s t-test). Error bars indicate s.e.m. (D) Proposed mechanism of suppression of translation initiation in tumor cells. MAPK/ERK and AKT/S6K1 are two major signaling pathways downstream of RAS. Blockage of both pathways results in effective suppression of eIF4B phosphorylation and inhibits translation initiation in proliferating tumor cells. |
|
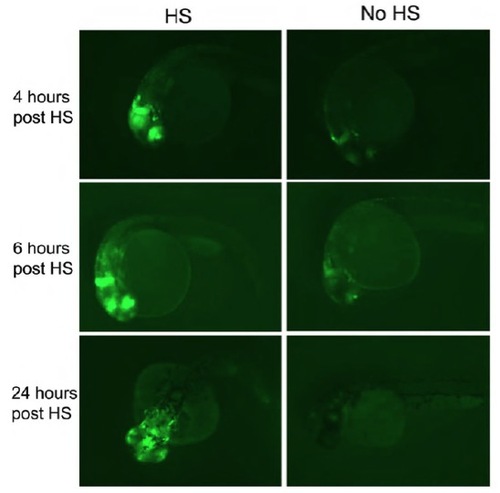
dusp6 activation in Tg(hsp70-HRASG12v; dusp6-d2EGFP) embryos with and without heat shock at different time points. Transgenic embryos were kept at 28.5°C until 22 hpf. Some received 37°C heat shock for 1 hour; some were kept as controls. At 4, 6 and 24 hours post-heat shock, EGFP expression was observed under a fluorescence microscope. Representative images for each time point for heat shocked and control are shown. |
|
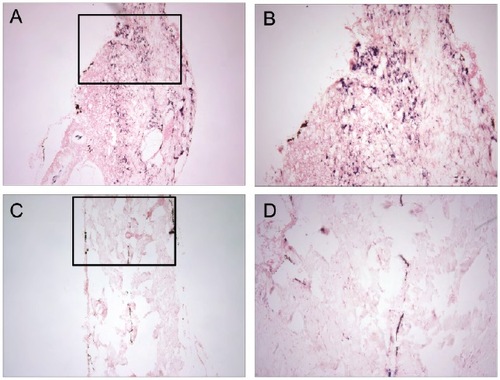
dusp6 expression is upregulated in zebrafish rhabdomyosarcoma. dusp6 expression was assessed by ISH. (A,B) Sections of zebrafish ERMS. Wild-type zebrafish embryos were injected with rag2-KRASG12D at the one-cell stage to induce rhabdomyosarcoma. (C,D) Sections of normal musculature. Wild-type siblings without injection as controls. (A,C) 10×, (B,D) 40×. |
|
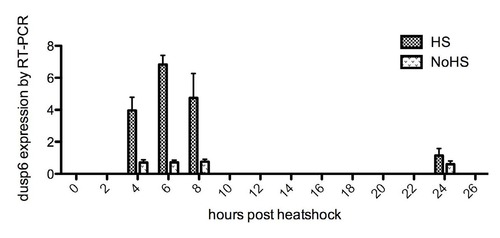
Quantitative RT-PCR analysis of dusp6 activation levels in Tg(hsp70-HRASG12V) at various time points after 1 hour of heat shock. Transgenic embryos were kept at 28.5°C until 22 hpf. Some received 37°C heat shock for 1 hour; some were kept as controls. At 4, 6, 8 and 24 hours post-heat shock, the embryos were harvested for RNA extraction and RT-PCR analysis. Transgenic embryos kept at 28.5°C without heat shock were used as controls. EXPRESSION / LABELING:
|
|
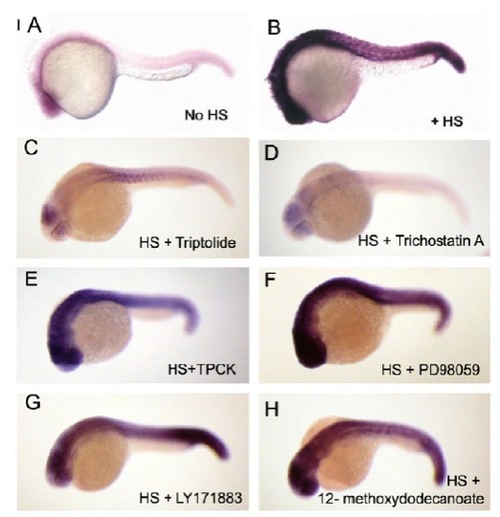
In situ hybridization of Cre in transgenic Tg(hsp70-Cre) embryos with chemical treatment. (A,B) Tg(hsp70-Cre) embryos with or without heat shock were fixed and subjected to ISH for Cre as controls. (C,D) Tg(hsp70-Cre) embryos were incubated in chemicals (Triptolide and TSA) from 22 hpf, heat shocked from 24-25 hpf, fixed at 30 hpf, and analyzed by ISH for Cre. The Cre expression levels were suppressed in comparison to B, indicating that these chemicals interfere with heat shock promoter activation or transcription. (E-H) Tg(hsp70-Cre) embryos were incubated in chemicals (TPCK, PD98059, LY171883 and 12-methoxydodecanoate) from 22 hpf, heat shocked from 24-25 hpf, fixed at 30 hpf, and analyzed by ISH for Cre. The Cre expression levels were not suppressed in comparison to B, indicating that these chemicals do not interfere with heat shock promoter activation or transcription; therefore, these chemicals were confirmed as suppressors of the RAS pathway. |
|
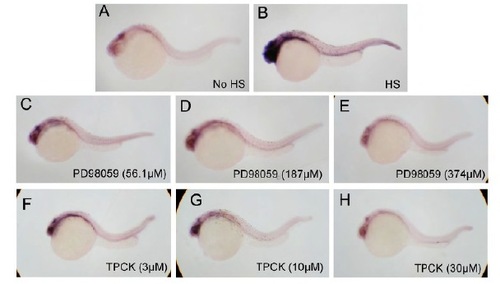
PD98059 and TPCK suppress dusp6 upregulation in Tg(hsp70-HRASG12V) embryos in a dose-dependent manner. dusp6 expression was assessed by ISH. (A,B) Tg(hsp70-HRASG12V) receiving heat shock or no heat shock treatment provided positive and negative controls. (C-E) Tg(hsp70-HRASG12V) receiving PD98059 treatment in an increasing dose demonstrated a decreasing expression level of dusp6. (F-H) Tg(hsp70-HRASG12V) receiving TPCK treatment in an increasing dose demonstrated a decreasing expression level of dusp6. |