- Title
-
JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Authors
- Drerup, C.M., and Nechiporuk, A.V.
- Source
- Full text @ PLoS Genet.
|
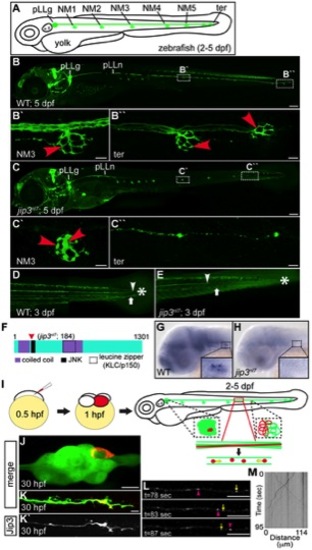
Jip3, an actively transported protein, was necessary for axon extension and the prevention of axon terminal swellings. (A) Schematic of a larval zebrafish illustrating the basic anatomy of the primary posterior lateral line (pLL) system. Neuromasts (NMs; terminal NM cluster-ter) are innervated by the pLL nerve (green), which emanates from the pLL ganglion (pLLg). (B) Wildtype neurod:EGFP transgenic at 5 dpf with the pLLg and pLL nerve (pLLn) indicated. (B′,B′′) Panels illustrate pLL axon terminals that innervate NM3 (B′) and the distal end of the pLL nerve including the axon terminals at the terminal NM cluster (ter; B′′; red arrowheads point to axon terminals). (C) jip3nl7 mutants displayed truncated pLL nerves and distal pLL nerve thinning (C′′) as well as swollen axon terminals in all NMs (NM3 shown in C2). Scale bars B and C = 100 μm. Scale bars in B′, B′′, C′ and C′′ = 10 μm. (D, E) Long central nervous system axons of the reticulospinal tract (arrowhead) and pLL efferent axons (arrow), visualized by the phox2b:EGFP transgenic reporter, were also truncated in jip3nl7 mutants. End of trunk indicated by the asterisk. (F) Schematic of the zebrafish Jip3 protein showing conserved structural and binding domains. The red arrowhead indicates the location of the jip3nl7 mutation, which generates a premature stop codon at amino acid 184. (G,H) In situ hybridization analysis revealed that jip3 was expressed in the central and peripheral nervous systems at 2 dpf in wildtype but was lost in jip3nl7. (I) Schematic of the paradigm designed to image axon transport in the pLL nerve. (J) Transient expression of Jip3-mCherry in 1 neuron of the pLL ganglion at 30 hpf. (K) Jip3-mCherry was localized to a growth cone of an extending axon at 30 hpf. The pLL ganglion and nerve were visualized by expression of the neurod:EGFP transgene. (L) Jip3-mCherry is actively transported in pLL axons (Video S1). Arrowhead (pink) and arrow (yellow) indicate anterograde and retrograde particle movement respectively. (M) Kymograph of time-lapse imaging in J. Scale bars in J–L = 10 μm. |
|
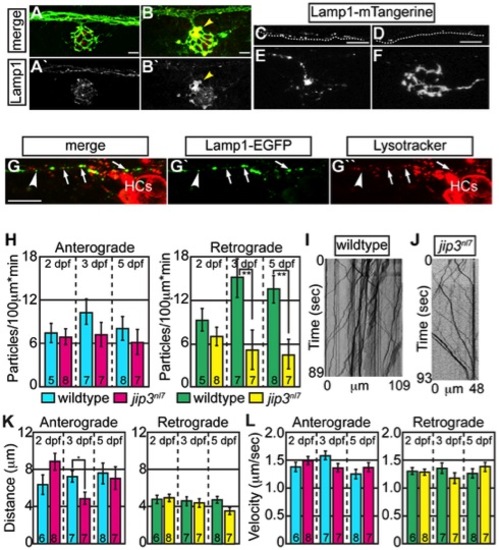
Lysosomes accumulated in jip3nl7 axon terminal swellings. (A,B) Lysosome density, as assayed by Lamp1 immunolabeling (A,B-red, A′B′-white) was increased in jip3nl7 NM3 axon terminals at 5 dpf (arrowhead). Larvae carried the neurod:EGFP transgene to label pLL axons. (C,D) Stills from imaging sessions of Lamp1-mTangerine transport in the pLL nerve of wildtype (C) and jip3nl7 mutant (D) embryos at 3 dpf (Videos S4 and S5). Dotted lines denote the lower bound of the axons imaged. (E,F) Lamp1-mTangerine accumulated in the axon terminals of jip3nl7 mutants (F) but not wildtype siblings (E) at 3 dpf (NM1 shown for both). (G) The majority of Lamp1-EGFP positive vesicles in axons co-labeled with Lysotracker red, indicating they were lysosomes. Arrows denote a subset of the Lamp1-EGFP/Lysotracker red co-labeled vesicles. Arrowhead points to a small Lamp1 positive vesicle that was not acidified. HC denotes hair cells rich in Lysotracker red positive, acidic vesicles. (H) Retrograde, but not anterograde, lysosome transport frequency was decreased in jip3nl7 mutants at 3 and 5 dpf (Wilcoxon rank-sum; **-p<0.005). Number of embryos analyzed is indicated on the graph for this and all subsequent bar graphs. (I,J) Kymographs of wildtype (I) and jip3nl7 (J) Lamp1-mTangerine transport shown in C and D. (K,L) Neither distance moved in individual bouts (K) nor velocity of movement (L) were altered in jip3nl7 mutants, save a decrease in anterograde transport distance at 3 dpf (Wilcoxon rank-sum; *p<0.05). Scale bars = 10 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
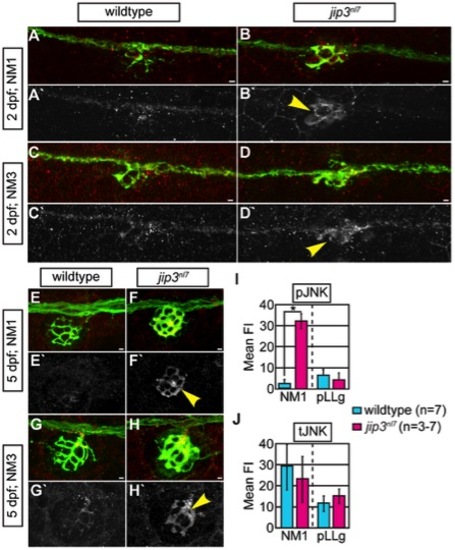
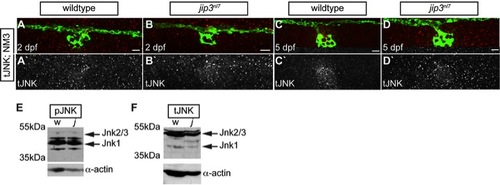
pJNK levels were elevated in jip3nl7 axon terminals. (A–H) Immunolabeling for active JNK (pJNK; red in merge; white in single channel) in proximal (NM1) and distal (NM3) neuromasts at 2 and 5 dpf. pJnk levels were elevated in all axon terminals in jip3nl7 mutants (A–I; arrowheads). (I,J) Mean fluorescent intensity (background subtracted; see Materials and Methods for details) of pJNK and total JNK (tJNK) labeling in NM1 axon terminals and the pLL ganglion (pLLg) at 5 dpf. (ANOVA, post-hoc contrasts; *-p<0.001). Scale bars = 10 μm. |
|
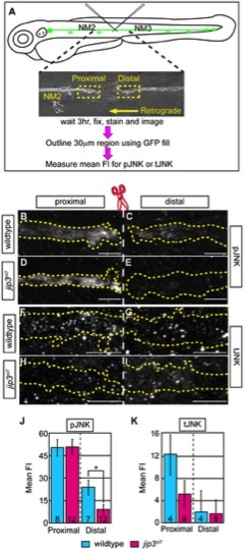
pJNK failed to accumulate distal to injury. (A) Schematic and time-line of the injury model experiment and fluorescent intensity quantification. pLL nerve (identified using the neurod:EGFP transgene) was severed using finely pulled glass capillaries. DIC image of a representative injury illustrates peripheral tissue remained mostly intact. Three hours post-injury, larvae were fixed and stained for GFP (to identify the nerve) and either pJNK or tJNK. Thirty µm areas immediately proximal or distal to the injury were imaged and the mean fluorescent intensity of pJNK or tJNK was determined in summed projected stacks through the nerve only in areas that overlapped with GFP expression (outlined by dotted lines in B–I). Background mean fluorescent intensity was determined in adjacent tissue. (B–I) Proximal and distal nerve (dotted outline) adjacent to site of injury (dashed line) in wildtype and jip3nl7 larvae immunolabled for pJNK (B–E) and tJNK (F–I). (J) Levels of pJNK were decreased distal to nerve injury in jip3nl7 but proximal levels were comparable to wildtype (ANOVA, post-hoc contrasts; *-p<0.05). (K) tJNK levels trended towards a decrease proximal to the site of injury in jip3nl7 (ANOVA, post-hoc contrasts; p<0.1790) but were not different in the retrograde pool, distal to axonal severing. PHENOTYPE:
|
|
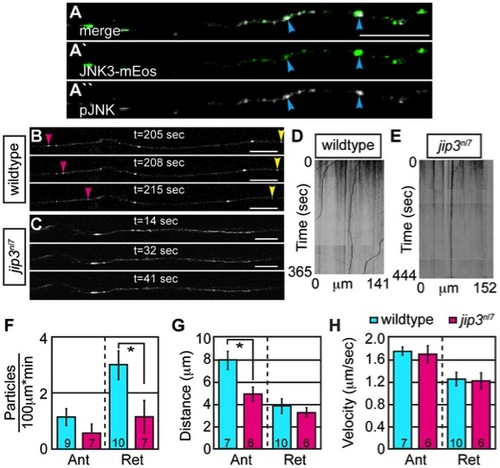
Retrograde JNK3 transport frequency was decreased in jip3nl7 mutants. (A) Immunolabeling for pJNK in an axon expressing JNK3-mEos showed a high degree of colocalization (arrowheads) indicating that a large percentage of axonal JNK3-mEos is activated. (B,C) Representative stills from a live imaging session showing axonal transport of JNK3-mEos in a pLL axon of a wildtype (B) and jip3nl7 mutant (C) at 2 dpf (see Videos S6 and S7). Pink arrowhead denotes anterograde movement, yellow retrograde movement. (D,E) Kymographs generated from these imaging sessions. (F) Number of retrograde JNK3-mEos puncta (corrected for size of analyzed region and time of imaging session) was decreased in jip3nl7 (ANOVA, post-hoc contrasts; *-p<0.05). Distance of individual retrograde movement bouts (G) and velocity (H) were unaltered in jip3nl7. Anterograde transport distance was decreased (*-p<0.05; Ant = anterograde; Ret = retrograde). Scale bars = 10 μm. PHENOTYPE:
|
|
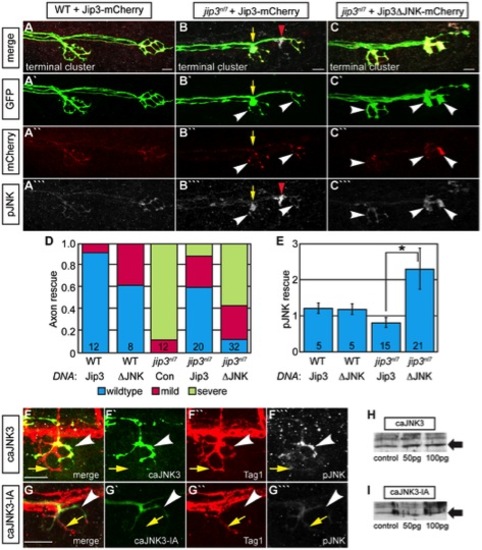
Jip3 interaction with JNK was necessary for pJNK clearance and the prevention of axon swellings. (A–C) Axon terminal swellings and pJNK accumulation were rescued by Jip3 but not by Jip3ΔJNK. Axonal swellings were visualized live by neurod:EGFP transgene expression; following live imaging, pJNK was assayed by individual larva immunolabeling at 4 dpf. White arrowheads mark axon terminals expressing the DNA constructs. Yellow arrow points to a swelling in an axon not expressing Jip3-mCherry. Red arrowhead denotes an underlying pJNK positive cell not expressing Jip3-mCherry. (D) Ratio of axon terminal swellings in each class (mild = small swellings, severe = large swellings) show rescue of axonal swellings by full-length Jip3 but not Jip3ΔJNK. DNA indicates the rescue construct injected; Jip3 = full-length Jip3-mCherry; ΔJNK = Jip3ΔJNK-mCherry; Con = uninjected control. Embryo genotype, determined by control axon terminal morphology, is indicated below each bar. (E) Ratio of pJNK levels in Jip3 or Jip3ΔJNK expressing axon terminals to those not expressing the rescue construct at 4 dpf. Jip3, but not Jip3ΔJNK, suppressed increased pJNK levels in jip3nl7 (Wilcoxon rank-sum; *-p<0.01). (F) Induction of constitutively active JNK3 tagged with EGFP (caJNK3; green) for 15 hours at 4 dpf increased the level of pJNK immunofluorescence concomitant with the induction of swellings shown by both the caJNK3-EGFP fill and Tag1 immunolabeling of neuronal membranes. Arrowhead points to a caJNK3-EGFP expressing axon. Yellow arrow indicates an axon terminal in the same NM not expressing this construct. (G) Axon terminal swellings were absent in axon terminals expressing an inactive form of the same construct (caJNK3-IA), indicating that JNK activation was necessary to induce swellings. (H,I) Efficacy of both caJNK3 and caJNK3-IA were assayed by Western blot analysis of phospho-cJun, a downstream target of active JNK. While whole embryo overexpression of caJNK3-EGFP by RNA injection induced elevated levels of phospho-cJun at 24 hpf (H), similar expression of the inactive form of caJNK3-EGFP (caJNK3-IA) failed to induce a similar increase (I). Scale bars = 10 μm. |
|
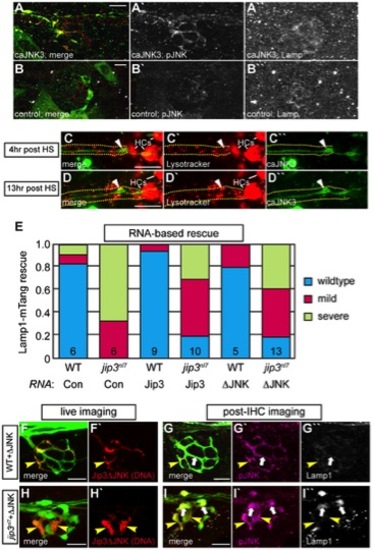
Increased levels of pJNK did not cause lysosome accumulation in jip3nl7. (A) Induction of caJNK3-EGFP at 4 dpf increased the level of pJNK immunofluorescence (middle) in a subset of axon terminals but did not lead to lysosome accumulation as compared to control (B). Scale bars = 10 μm. (C, D) This result was confirmed by Lysotracker red labeling. Surrounding, non-caJNK3-EGFP positive axons show similar numbers, size and density of lysosomes both 4 hours and 13 hours after induction of caJNK3. The pLL nerve was visualized by phase contrast optics and is outlined. Arrowhead indicates axonal swellings caused by high levels of activated JNK. HC denotes neuromast hair cells that strongly label with Lysotracker red. (E) Whole embryo expression of Jip3 and Jip3ΔJNK by mRNA injection partially suppressed the accumulation of lysosomes in jip3nl7 mutant axon terminals at 3 dpf as assayed by expression of Lamp1-mTangerine in pLL neurons. Wildtype – Lamp1-mTangerine positive small puncta only; Mild – small puncta and aggregates visible; Severe - few to no small puncta apparent and large aggregations of Lamp1-mTangerine. (F–I) Injection of 10 pg of a DNA construct encoding Jip3ΔJNK-mCherry rescued lysosome accumulation in jip3nl7 axon terminals. Larvae that expressed Jip3ΔJNK-mCherry (red) in pLL axons and carried the neurod:EGFP transgene were first imaged live (F,H) to identify expressing axon terminals. They were then individually fixed, stained for pJNK (pseudo-colored magenta) and Lamp1 (white), and subsequently the same axon terminals were reimaged (G,I). Arrowheads point to axon terminals in wildtype (F,G; NM1) and jip3nl7 (H,I; NM5) that express Jip3ΔJNK-mCherry (red) at 5 dpf. Arrows point to axon terminals in the same NMs that did not express this construct. Note that expression of Jip3ΔJNK-mCherry in jip3nl7 completely rescued lysosome accumulation (yellow arrowheads in I′′) but failed to rescue high levels of pJNK (yellow arrowheads in I′). |
|
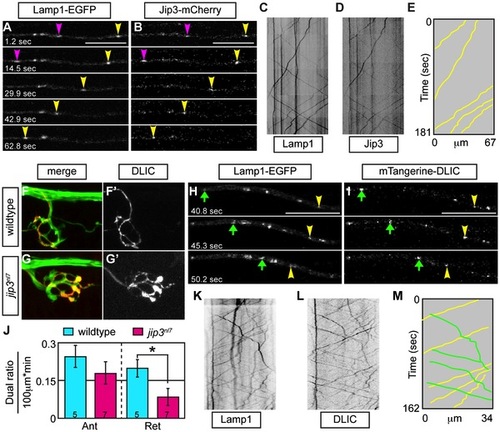
Jip3 scaffolds lysosomes to DLIC for retrograde transport. (A,B) Stills from a wildtype imaging session at 3 dpf in which Lamp1-EGFP (A) and Jip3-mCherry (B) co-transport was analyzed (Video S10). Pink and yellow arrowheads point to two retrograde Jip3/Lamp1 positive cargos. (C,D) Kymographs generated from this imaging session for individual cargos. (E) Schematized kymograph of co-transport. Yellow lines denote Jip3-positive lysosomes moving in the retrograde direction. (F,G) mTangerine-DLIC expression in a wildtype (F) and jip3nl7 mutant (G) NM1 axon terminal at 3 dpf. (H,I) Stills from analysis of Lamp1 (H) and DLIC (I) co-transport at 3 dpf in a wildtype (Video S11). Green arrow-anterograde co-labeled puncta. Yellow arrowhead-DLIC positive lysosome undergoing retrograde transport. (J) The ratio of DLIC positive lysosomes moving in the retrograde direction was significantly decreased in jip3nl7 mutants (ANOVA, *-p<0.05; Anterograde-Ant; Retrograde-Ret). (K–M) Kymographs from this imaging session and schematized kymograph depicting co-labeled anterograde lysosomes in green and retrograde in yellow. PHENOTYPE:
|
|
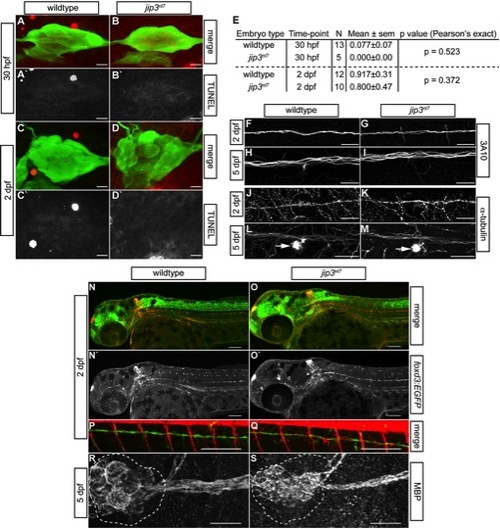
pLL nerve abnormalities in jip3nl7 were not due to cell death, general cytoskeletal defects or glial deficits. (A–D) Cell death in the pLL ganglion was examined by TUNEL assay at 30 hpf and 2 dpf. Confocal projections of the pLL ganglion showed that TUNEL labeling (red in merge, white in single channel) was not elevated in jip3nl7 during (30 hpf) or after (2 dpf) nerve extension. Expression of the neurod:EGFP transgene marks the pLL ganglion. (E) Quantification of TUNEL-positive cells in the pLL ganglia of wildtype and mutant embryos. (F–I) Immunolabeling with an antibody against a neurofilament associated antigen demonstrated no deficit in the pLL nerve of jip3nl7 at 2 and 5 dpf. (J–M) Similarly, analysis of microtubule density in the pLL nerve with an antibody against α-tubulin revealed no significant changes in the jip3nl7. Arrow indicates hair cell stereocilia which contain high levels of tubulin. The distal nerve at NM4 is shown for F–M. (N–Q) Glial cell occupation of the pLL nerve was analyzed using a combination of two transgenic lines, TgBAC(foxd3:EGFP)nl5 (glia) and TgBAC(neurog1:DsRed)nl6 (nerve). Just after nerve extension (2 dpf), glial occupation of the pLL nerve was normal in jip3nl7. The distal portion of the pLL nerve at NM4 is shown in P and Q. (R,S) Myelination of the pLL nerve was also normal at 5 dpf in jip3nl7 as assayed by antibodies against MBP (myelin basic protein). The pLL ganglion is outlined. Scale bars in A–D, F–M and R,S are 25 μm; N–Q are 100 μm. |
|
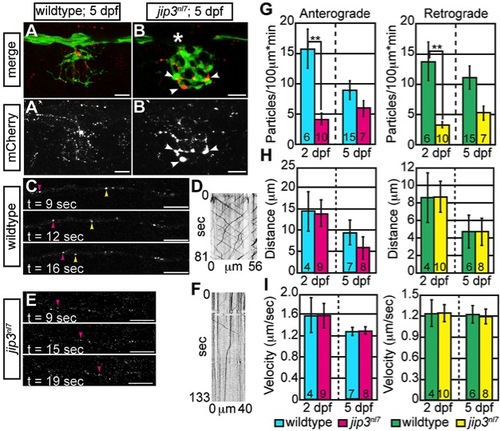
Membrane-bound cargo accumulated in jip3nl7 axon terminals due to failed retrograde transport. (A,B) ssNPY-mCherry (red) accumulated in mutant (arrowheads), but not wildtype axon terminals at 5 dpf. Asterisk indicates portion of the nerve occluded by a pigment cell. The pLL axons were visualized by expression of the neurod:EGFP transgene. (C–F) Representative still images from Videos S2 and S3 and kymographs from transport analysis in 5 dpf larvae. (G) Analysis of anterograde and retrograde ssNPY-mCherry particle movements at 2 dpf revealed a decrease in anterograde and retrograde cargo movement (ANOVA; **-p<0.005). (H, I) Other transport parameters, including distance moved in individual movement bouts and movement velocity, were unchanged in jip3nl7. Scale bars = 10 μm. Number of embryos analyzed is indicated on each bar of the graph. |
|
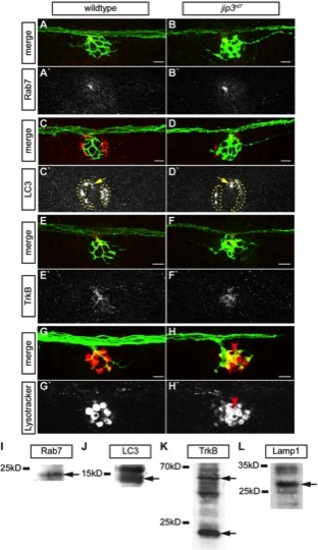
Lysosomes, but not late endosomes, signaling endosomes or autophagosomes, accumulated in jip3nl7 axon terminals. (A–H) The density of late endosomes, autophagosomes, TrkB, and lysosomes were assayed in wildtype and jip3nl7 axon terminals by immunolabeling (A–F) and live imaging (G,H). (A,B) Rab7, a marker of late endosomes, was unchanged in jip3nl7 axon terminals. (C,D) LC3, a marker of autophagosomes, was unchanged in jip3nl7 axon terminals (yellow arrow). Note the high LC3 expression in NM support cells (dotted outline). (E,F) TrkB levels were slightly decreased in jip3nl7 axon terminals. (G,H) Lysotracker red staining at 5 dpf revealed elevated levels of lysosomes in jip3nl7 axon terminals (red arrowhead). Asterisk points out representative hair cells. This cell type has high Lysotracker labeling due to the large number of acidic vesicles. (I–L) Western blot analyses of 3 dpf embryo lysates demonstrate the specificity of the antibodies used. Arrows indicate bands corresponding to those that match the size of the predicted zebrafish protein orthologs. |
|
Total JNK levels were not elevated in jip3nl7. (A–D) Total JNK (tJNK) levels were unchanged in jip3nl7axon terminals at 2 and 5 dpf. (E,F) Western blot analysis of 3 dpf whole embryo extracts indicated overall levels of pJNK (E) and tJNK (F) were not changed in jip3nl7 (j) compared to wildtype (w). α-actin control below. Scale bars = 10 μm. |
|
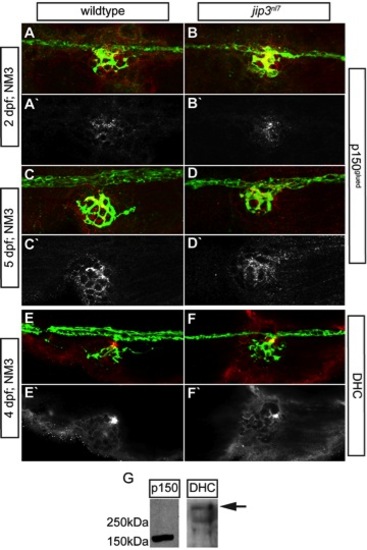
Components of the dynein retrograde motor complex were present at jip3nl7 axon terminals. (A–E) Immunolabeling for two components of the dynein motor complex, p150glued (A–D; 2 and 5 dpf) and dynein heavy chain (E,F; 4 dpf) demonstrated that levels and distribution of these dynein motor components in axon terminals were similar between jip3nl7 and wildtype controls. neurod:EGFP transgene carriers were used to label the pLL nerve (green). The middle portion of the pLL nerve at NM3 is shown in A–F. (G) Western blot analysis of 4 dpf whole embryo extracts demonstrated the specificity of the respective antibodies (arrow indicates DHC band based on predicted molecular weight). |
|
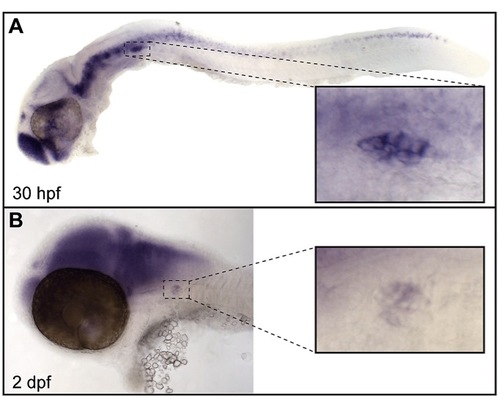
jnk3 is expressed in the peripheral and central nervous systems of the zebrafish embryo. (A) In situ hybridization for jnk3 at 30 hpf revealed expression in the central and peripheral nervous system, including the pLL ganglion (inset). (B) jnk3 expression in these regions, including the pLL ganglion (inset), persisted at 2 dpf. |