- Title
-
Zebrafish second heart field development relies on progenitor specification in anterior lateral plate mesoderm and nkx2.5 function
- Authors
- Guner-Ataman, B., Paffett-Lugassy, N., Adams, M.S., Nevis, K.R., Jahangiri, L., Obregon, P., Kikuchi, K., Poss, K.D., Burns, C.E., and Burns, C.G.
- Source
- Full text @ Development
|
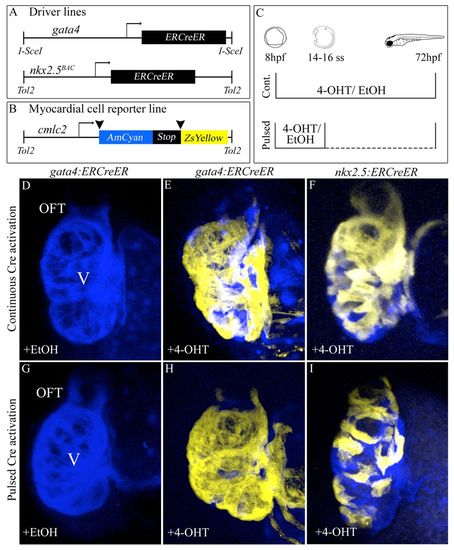
Specification of gata4+ and nkx2.5+ SHF myocardial progenitors in the zebrafish ALPM. (A,B) Driver and reporter transgenes employed for inducible Cre/loxP-mediated myocardial lineage tracing of gata4+ and nkx2.5+ ALPM cells. (C) Experimental strategy. Double transgenic embryos were exposed continuously (’Cont.’) to 10 µM 4-OHT or solvent alone (ethanol) between 8 and 72 hpf. Alternatively, embryos were exposed transiently (’Pulsed’) to 4-OHT or ethanol between 8 hpf (75% epiboly) and the 14- to 16-somite stages prior to extensive rinsing with embryo medium (E3). All embryos were analyzed and imaged at 72 hpf for cardiac ZsYellow reporter fluorescence. (D,G) Hearts in Tg(gata4:ERCreER); Tg(cmlc2:CSY) embryos treated continuously (D) or transiently (G) with ethanol. (E,H) Hearts in Tg(gata4:ERCreER); Tg(cmlc2:CSY) embryos treated continuously (E) or transiently (H) with 4-OHT. (F,I) Hearts in Tg(nkx2.5:ERCreER); Tg(cmlc2:CSY) embryos treated continuously (F) or transiently (I) with 4-OHT. Hpf, hours post-fertilization; ss, somite stage; V, ventricle; OFT, outflow tract; EtOH, ethanol; 4-OHT, 4-hydroxytamoxifen. |
|
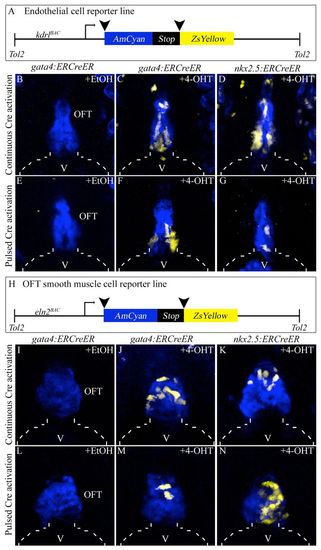
Specification of gata4+ and nkx2.5+ SHF endothelial and smooth muscle progenitors in the zebrafish ALPM. (A) Schematic of the endothelial/endocardial reporter transgene. (B-G) OFT regions of 6 dpf double transgenic embryos treated continuously (8-72 hpf; B-D) or transiently (75% epiboly to 14- to 16-somite stages; E-G) with ethanol (B,E) or 4-OHT (C,D,F,G). (H) Schematic of the OFT smooth muscle reporter transgene. (I-N) OFT regions of 6 dpf double transgenic embryos treated continuously (8-72 hpf; I-K) or transiently (75% epiboly to 14-16 ss; L-N) with ethanol (I,L) or 4-OHT (J,K,M,N). Broken lines outline the ventricle (V). EtOH, ethanol; OFT, outflow tract; 4-OHT, 4-hydroxytamoxifen. |
|
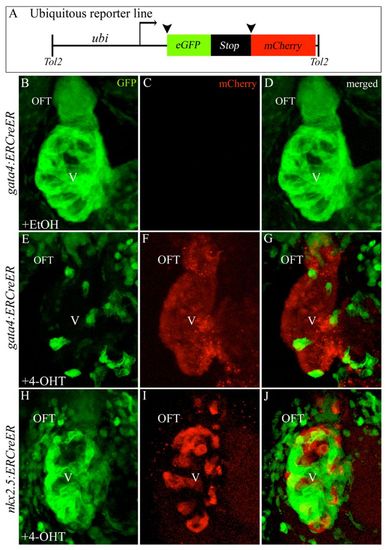
Ubiquitous reporter transgene corroborates specification of gata4+ and nkx2.5+ SHF progenitors in the ALPM. (A) Ubiquitous reporter transgene. (B-J) Hearts in 72 hpf double transgenic embryos treated transiently (75% epiboly to 14- to 16-somite stages) with ethanol [B-D; n=0/8 double transgenic embryos with cardiac mCherry reporter fluorescence; for nkx2.5 trace (hearts not shown), n=0/17 with reporter fluorescence] or 4-OHT (E-J) imaged in the green (B,E,H) and red (C,F,I) channels. Merged images are shown in D,G,J. n=18/18 double transgenic embryos with cardiac mCherry reporter fluorescence for E-G. n=15/15 with reporter fluorescence for H-J. OFT, outflow tract; V, ventricle; EtOH, ethanol; 4-OHT, 4-hydroxytamoxifen. |
|
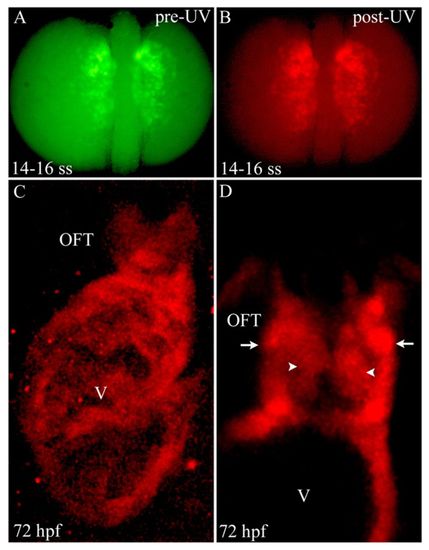
Photoconversion of nkx2.5+ cells in the ALPM reveals widespread contributions to SHF-derived structures. (A,B) Dorsal images of a 14- to 16-somite stage Tg(nkx2.5:Kaede) embryo before (A) and after (B) photoconversion with ultraviolet (UV) light. (C) Confocal z-stack image of the ventricle (V) and OFT at 72 hpf. (D) Confocal slice of the OFT. Arrowheads and arrows highlight endothelial and smooth muscle precursor cells in the OFT, respectively. Six out of eight photoconverted embryos exhibited red Kaede protein throughout the ventricle and OFT. ss, somite stage; OFT, outflow tract; hpf, hours post-fertilization. |
|
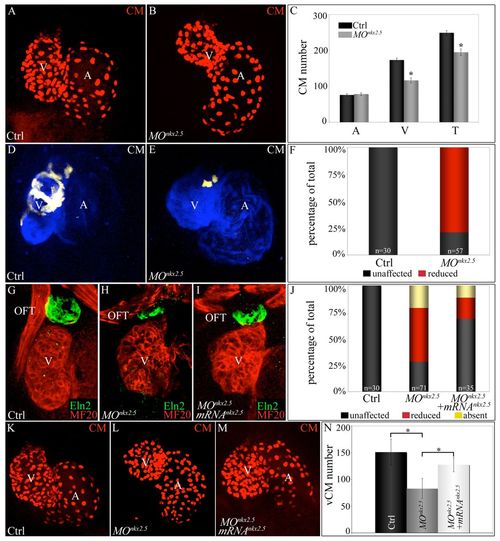
Morpholino-mediated inhibition of nkx2.5 pre-mRNA splicing elicits SHF phenotypes. (A-C) nkx2.5 morphants display ventricular cardiomyocyte deficits. z-stack images of hearts in control (A) and nkx2.5 morpholino-injected (B) Tg(cmlc2:dsRed2-nuc) embryos imaged at 72 hpf. (C) Average numbers of atrial (A), ventricular (V) and total (T) cardiomyocytes in control (n=6) and morphant (n=11) embryos. Error bars represent one s.d. *P<0.001. (D-F) SHF-derived ventricular myocardium is specifically reduced in nkx2.5 morphants. Control (D) and morpholino-injected (E) Tg(ltbp3:TagRFP2Acre); Tg(cmlc2:CSY) embryos imaged in the blue and yellow channels (merged images shown) by confocal microscopy at 72 hpf. (F) The percentages of embryos with reduced ventricular ZsYellow expression. The n values are reported in the graph. (G-J) nkx2.5 morphants exhibit reductions in Eln2+ OFT cells, a phenotype partially rescued by co-injection of nkx2.5 mRNA. Control (G; unaffected), morphant (H; reduced) and mRNA-injected morphant (I; unaffected) embryos were co-stained at 72 hpf with MF20 and anti-Eln2 antibodies to label myocardium (red) and OFT smooth muscle (green), respectively. (J) Percentages of embryos with unaffected, reduced or absent Eln2 staining. The n values are reported in the graph. (K-N) Injection of nkx2.5 mRNA partially rescues the ventricular cardiomyocyte deficit in nkx2.5 morphants. Control (K), morphant (L) and mRNA-injected morphant (M) Tg(cmlc2:dsRed2-nuc) embryos imaged by confocal microscopy at 72 hpf. (N) Average numbers of ventricular cardiomyocytes (vCMs) in each experimental group (n=4 per cohort). Error bars represent one s.d. *P<0.05. CM, cardiomyocytes; V, ventricle; A, atrium; OFT, outflow tract; Ctrl, control; MO, morpholino. |
|
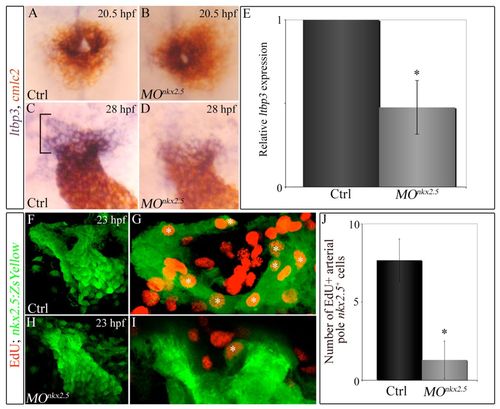
Knocking down nkx2.5 compromises arterial-pole ltbp3 expression and SHF proliferation. (A-D) Double in situ hybridization of cmlc2 and ltbp3 transcripts in control (A,C) and morphant (B,D) embryos at 20.5 (23-somite stage, cardiac cone stage; n=20, control; n=32, morphant) and 28 (linear heart tube stage; n=20, control; n=30/48 morphants) hpf. Bracket highlights non-myocardial ltbp3+ SHF progenitors. (E) Graph showing the average relative abundance of ltbp3 transcripts in morphant compared with control embryos at 28 hpf determined by quantitative PCR. Error bar represents one s.d., *P<0.05. (F-J) nkx2.5 morphants exhibit reductions in proliferating arterial-pole nkx2.5+ SHF cells. At 23 hpf, control and morphant Tg(nkx2.5:ZsYellow) embryos were double immunostained for incorporated EdU (red) and ZsYellow protein (green). Confocal z-stack images of heart tube regions in control (F,G) and morphant (H,I) embryos captured through green and red filters. (F,H) Flattened z-stack images (green channel only) of representative control (F) and morphant (H) heart tubes. (G,I) Arterial poles of heart tubes shown in F and H. Three contiguous confocal slices (red and green channels merged) from F and H were flattened. White asterisks mark double-positive cells (EdU+ red nuclei surrounded by ZsYellow+ green cellular fluorescence). (H) Average numbers of double-positive cells in control and morphant heart tubes. n=6 embryos per experimental group. Error bars represent one s.d., *P<0.0001. hpf, hours post-fertilization; Ctrl, control; MO, morpholino. |
|
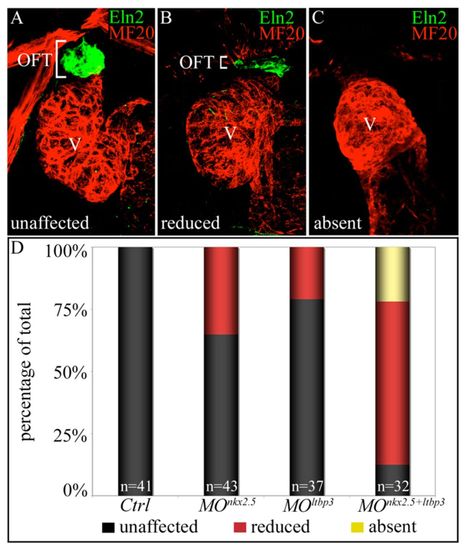
nkx2.5 and ltbp3 interact genetically during zebrafish SHF development. (A-C) nkx2.5 and ltbp3 morpholinos were injected alone or together into one-cell stage embryos. Total morpholino mass remained constant between experimental groups (see Materials and methods). Injected embryos were processed at 72 hpf for MF20 (myocardium; red) and Eln2 (OFT smooth muscle; green) double immunostaining. Embryos were binned into three phenotypic classes, unaffected (A), reduced (B) or absent (C), based on Eln2 OFT signal. White brackets highlight Eln2 staining. (D) Percentages of embryos in each phenotypic class according to experimental group. The n values are reported in the graph. OFT, outflow tract; V, ventricle; Ctrl, control; MO, morpholino. |
|
ALPM expression of ZsYellow protein in Tg(nkx2.5:ZsYellow) embryos. (A, B) 12-somite (A) and 14-somite (B) stages. |
|
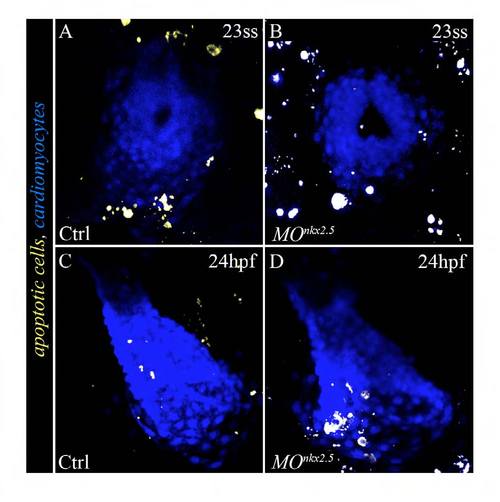
SHF progenitors do not undergo aberrant apoptosis in nkx2.5 morphants. (A-D) Confocal images of hearts in 23-somite stage(A,B) and 24 hpf (C,D) control (n=67) and morphant (n=70) embryos ubiquitously expressing the yellow fluorescent apoptosis reporter secA5-YFP (van Ham et al., 2010). Myocardial cells are labeled with a blue fluorescent protein (cerulean). ss, somite stage; hpf, hours postfertilization; Ctrl, control; MO, morpholino. |