- Title
-
Stretching morphogenesis of the roof plate and formation of the central canal
- Authors
- Kondrychyn, I., Teh, C., Sin, M., and Korzh, V.
- Source
- Full text @ PLoS One
|
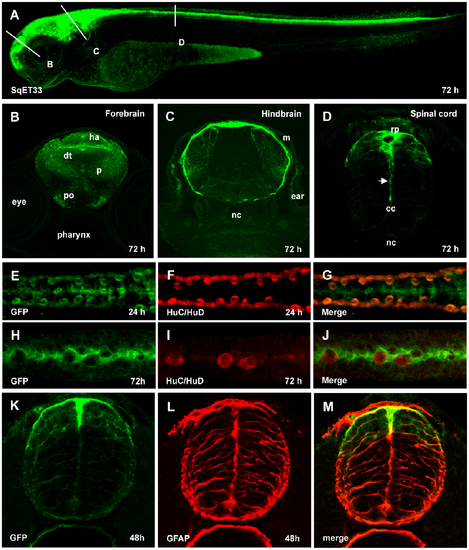
Characterization of SqET33 transgenic line. (A), Confocal image of 3 dpf larva of SqET33 line, lateral view. Dashed lines depict the position of transverse sections shown in B–D. (B–D), transverse sections, immunofluorescent staining with anti-GFP antibodies. Arrow indicates the elongated central process of the roof plate cell. (E–J), whole-mount immunofluorescent staining with anti-GFP (midline RP cells and lateral dorsal interneurons) and anti-HuC/HuD (lateral dorsal neurons) antibodies, dorsal view of spinal cord. (K–M), immunofluorescent staining with anti-GFP (green) and anti-GFAP (red) antibodies, transverse section of the spinal cord. Abbreviations: cc, central canal; dt, dorsal thalamus; ha, habenula; m, meninx; nc, notochord; p, pallium; po, preoptic area; rp, roof plate. EXPRESSION / LABELING:
|
|
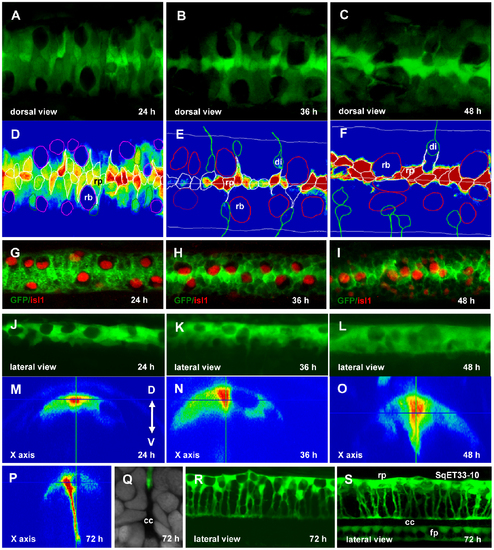
Re-orientation of the RP cells. Confocal images of the spinal cord of SqET33 line at different developmental stages (A–C, dorsal view, J–L, R, lateral view, M–P, orthogonal optical sections) and outline of the dorsal cells (D–F) superimposed onto the GFP intensity chart. Note that RB cells undergo the lateral-medial displacement. (G–I), Whole-mount immunohistochemistry detecting Islet1 in RB cells (red, nuclei), dorsal view of the spinal cord. (Q), Transverse section of the spinal cord at high magnification showing a fine structure of the central canal. RP process is stained with anti-GFP (green) and nuclei are counterstained with DAPI (grey). (S), Confocal image of the spinal cord of SqET33-10 line expressing GFP in the roof and floor plates, lateral view. Abbreviations: cc, central canal; di, dorsal interneurons; fp, floor plate; rb, Rohon-Beard cells; rp, roof plate cells. EXPRESSION / LABELING:
PHENOTYPE:
|
|
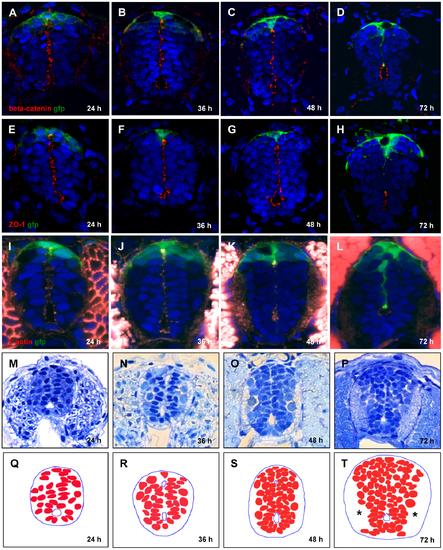
Conversion of primitive lumen into central canal. (A–L), Contraction of opposed apical surfaces reflects recession of the primitive lumen into a central canal between 24 and 72 hpf. Immunofluorescent staining by a combination of anti-GFP with anti-β-catenin antibody (A–D), anti-ZO-1 antibody (E–H), and phalloidin that detects F-actin (I–L). Increase in cell number and build-up of axonal tracts (*), plastic transverse sections of the spinal cord (M–P). (Q–T), Schematics corresponding to (M–P) showing cell nuclei. EXPRESSION / LABELING:
|
|
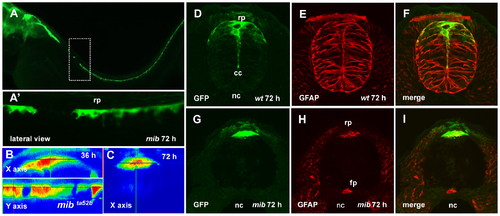
The roof plate formation in the mib mutant. (A), Confocal images, lateral view of the spinal cord of mib mutant, 72 hpf. Dashed rectangular shows the magnified in (A2) region of the spinal cord with the absence of GFP-positive RP cells. (B, C), the orthogonal optical sections of confocal images of the spinal cord of mib mutant illustrate lack of the roof plate extension between 36 and 72 hpf. Immunofluorescent staining of the transverse sections of spinal cord using anti-GFP (green) and anti-GFAP (red) antibodies; wild-type embryo (D–F) and mib mutant (G–I). EXPRESSION / LABELING:
PHENOTYPE:
|
|
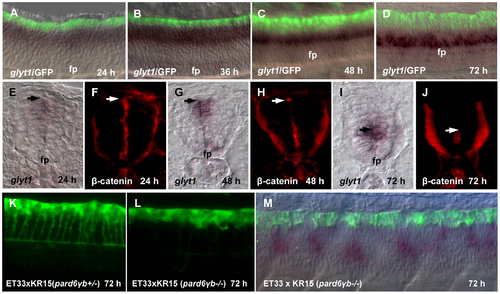
Growth of the RP processes correlates with a shift of glyt1 expression along D–V axis. (A-D), Double whole mount in situ hybridization (glyt1, dark purple) and immunostaining (GFP, green). glyt1 is expressed in the midline glial cells except RP; GFP is expressed in the RP cells. (E-J), Whole mount in situ hybridization (glyt1, dark purple) and immunostaining (β-catenin, red), the transverse sections of the spinal cord. The arrow shows an approximate position of attachment of the RP process to the apical surface of central canal. Confocal images of the spinal cord of pard6γb heterozygote (K) and pard6γb mutant (L) transgenic fish. (M), double whole mount in situ hybridization (glyt1, dark purple) and immunostaining (GFP, green) of pard6γb mutant. Abbreviation: fp, floor plate. EXPRESSION / LABELING:
PHENOTYPE:
|
|
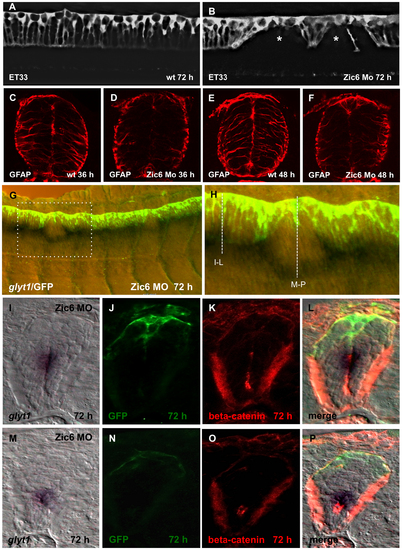
Zic6 is required for development of the radial glial scaffold and attachment of RP cells to the dorsal surface of central canal. Confocal images, lateral view of the spinal cord, 72 hpf. SqET33, control (A) and Zic6 morphant (B). The asterisk indicates the “gaps” in the palisade of RP cells. (C–F), Immunostaining of the transverse sections of the spinal cord with anti-GFAP antibody. (G), Double whole-mount in situ hybridization (glyt1, dark purple) and immunostaining (GFP, yellow/green) of the spinal cord Zic6 morphant. The dashed rectangular marks the magnified region (H). The dashed lines show the approximate positions for the transverse sections shown in (I–P). (I–P), distribution of glyt1, β-catenin and GFP in two positions in the spinal cord of Zic6 morphants shown in H, corresponding to I–L and M–P. EXPRESSION / LABELING:
PHENOTYPE:
|
|
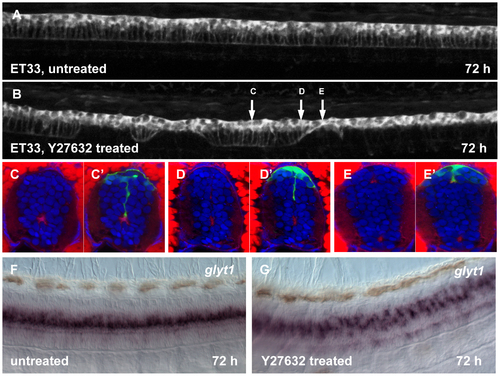
Effect of Rock inhibition on the RP morphogenesis. Confocal images of 72 hpf larva, untreated (A) and after Y-27632 injection into hindbrain ventricle at 30 hpf (B). The arrows show an approximate position of the transverse sections. (C–E2), transverse sections of the spinal cord of larva, treated with Y-27632. Immunofluorescent staining with anti-GFP (green) and phalloidin (red). Whole mount in situ hybridization with glyt1 antisense RNA probe, control (F) and after treatment with Y-27632 (G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
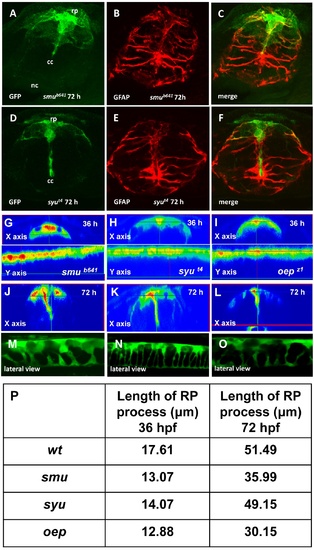
Mutant analysis of RP formation. Immunofluorescent staining of the transverse sections of the spinal cord of smu (A-C) and syu (D-F) mutants. Confocal images of the spinal cord of smu (M), syu (N) and oep (O) mutants at 72 hpf. Orthogonal optical sections of the confocal images of the spinal cord of smu (G, J), syu (H, K) and oep (I, L) mutants at different developmental stages. (P), The length of RP process in different mutants. PHENOTYPE:
|
|
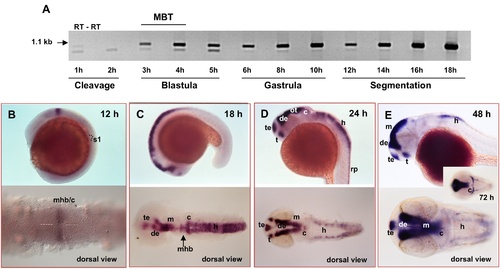
Expression pattern of zic6. (A), RT-PCR analysis of zic6 expression. Each even line represents negative control (minus RT). MBT, mid-blastula transition. (B–E), Whole-mount in situ hybridization with zic6 RNA probe at different developmental stage. c, cerebellum; de, diencephalon; h, hindbrain; m, midbrain; mhb, midbrain-hindbrain boundary; ot, optic tectum; rp, roof plate; s1, approximate position of the 1st somite; t, thalamus; te, telencephalon. |
|
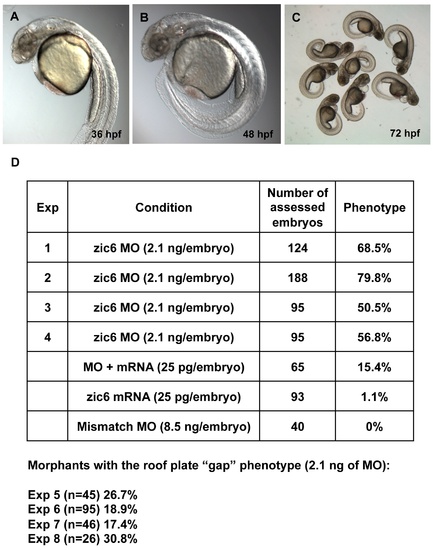
Zic6 morpholino knock-down experiments. (A–C), The morphants developed the curled-down body axis, the abnormal hindbrain reminiscent of that in mib mutants, cardiac edema. (D), Zic6 morpholino knock-down experiments (Exp 1 to 3) and rescue experiment (Exp 4). The roof plate “gap” phenotype was scored at 72 hpf in separate experiments (Exp 5 to 8, the numbers of assessed embryos are shown in brackets). |