- Title
-
On the embryonic origin of adult melanophores: the role of ErbB and Kit signalling in establishing melanophore stem cells in zebrafish
- Authors
- Dooley, C.M., Mongera, A., Walderich, B., and Nüsslein-Volhard, C.
- Source
- Full text @ Development
|
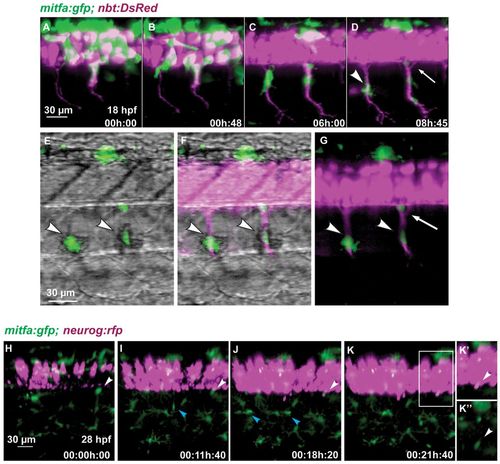
Melanoblasts migrate along the motor neurons. (A-G) Time-lapse confocal imaging of a Tg(mitfa:gfp;nbt:DsRed) zebrafish embryo. Medially migrating GFP-positive cells (arrowhead in D) migrate along ventrally extending primary motor axons (DsRed) starting at 18 hpf. A portion of the area in D is shown in G, and in E,F with brightfield illumination. Arrowheads in E-G indicate melanoblasts melanising in situ. A GFP-positive cell remains at a ventral position of the neural tube (arrow in D,G). (H-K′′)Tg(mitfa:gfp;-8.4neurog1:nrfp) embryo imaged starting at 28 hpf over a period of 22 hours. A GFP-positive cell (white arrowheads) located close to the ventral base of the neural tube remains at this position over the next 21 hours when the expression of nRFP marks the appearance of the DRG in the same region. A medially positioned cell (blue arrowhead in I) migrates out to the horizontal myoseptum and divides to form melanophores of the lateral stripe (blue arrowheads in J). (K′) Enlargement of the boxed region in K. (K′′) Green channel only. EXPRESSION / LABELING:
|
|
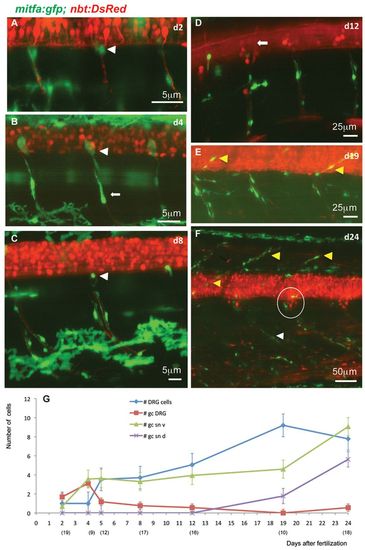
Stationary MPs located at the DRGs give rise to MPs located along the spinal nerves. (A-F) Time-lapse confocal imaging of Tg(mitfa:gfp;nbt:DsRed) zebrafish individuals during larval development of consecutive ages. GFP-positive cells at the exit point of the spinal nerves (white arrowheads in A-C) appear to be stationary. Other GFP-positive cells of more elongated shapes are observed along the spinal nerves (e.g. arrow in B). Until 12 dpf (D), no GFP-positive cells can be seen along the dorsally extending spinal nerves (arrow in D), whereas at metamorphic stages (E,F) both the dorsally extending (yellow arrowheads) and the ventrally extending (white arrowhead) spinal nerves show an increased number of associated GFP-positive cells. The number of cells in each DRG (circled in F) has increased to about eight. (G) Average numbers of GFP-positive cells per hemisegment located at the DRGs, the ventral and dorsal spinal nerves as well as DRGs at increasing larval age (dpf). The numbers of hemisegments counted is indicated below the respective time points. gc DRG, green cells at DRG; gc sn v, green cells at spinal nerves ventral; gc sn d, green cells at spinal nerves dorsal. EXPRESSION / LABELING:
|
|
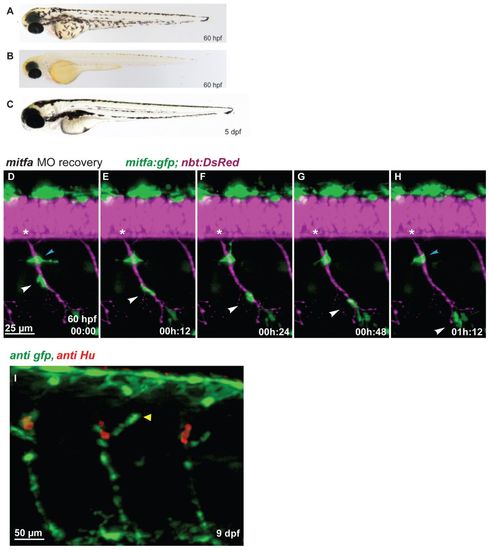
Regenerating MPs emerge at the site of the DRGs and travel along the spinal nerves. (A-C) In contrast to wild type (A), mitfa-MO-treated zebrafish embryos (B) lack larval melanophore pigmentation until 60 hpf, but regenerate the larval melanophore stripes completely by day 5 (C). (D-H) Tg(mitfa:gfp; nbt:DsRed) embryo previously treated with mitfa-MO imaged for several hours starting at 60 hpf (digital sectioning). A GFP-positive cells (white arrowhead) migrates along the spinal nerve. Another cell remained stationary (blue arrowhead) but was later observed to migrate away. Asterisks indicate the position of the DRG. (I) In mitfa morphant larvae (9 dpf), the regenerating MPs form a string of mitfa-positive cells along the spinal nerves associated with the DRGs both dorsally (arrowhead) and ventrally. |
|
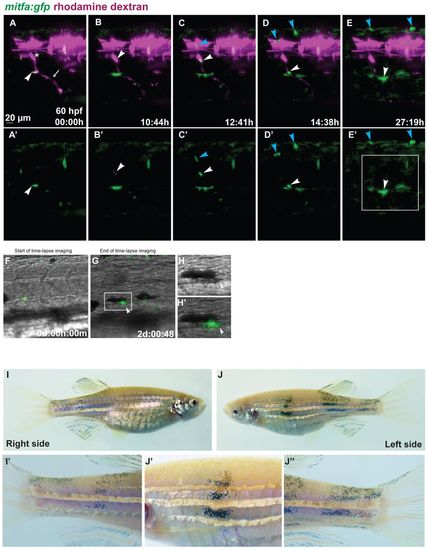
Tracing individual MPs during regeneration in chimeric animals. Blastula transplantations were performed with Tg(mitfa:gfp) zebrafish embryos injected with rhodamine dextran as donors and alb embryos injected with mitfa-MO as recipients. (A-E′) Red and green channel are shown in A-E, green channel only in A′-E′. Starting at 60 hpf (A,A′), we imaged a clone appearing at the site of a rhodamine-labelled DRG (asterisk) from which a peripheral axon extends ventrally (arrow). The arrowhead in A,A′ points to a GFP-positive cell located at the ventral side of the myotome. Another labelled cell appears at 70 hpf at the site of the DRG (arrowhead in B,B′). This cell divides, and the two daughter cells migrate dorsally (blue arrowhead) and ventrally (white arrowhead) along a spinal nerve (C,C′). The dorsally migrating cell divides once (blue arrowheads in D-E2), its progeny arrive at the dorsal side of the larva. The ventrally migrating cell divides once (white arrowheads in D-E2). (F-H′) The daughter cells, after reaching a position along the ventral stripe close to a cell that arrived there earlier, melanise (arrowheads in G-H′). Complete time-lapse imaging is shown in supplementary material Movies 2, 3. The region marked in E′ is shown in more detail in G, and the boxed area in G is enlarged in H,H′. (I-J′′) Chimeric adult fish that had developed several donor-derived melanophores at the lateral stripe in late larval stages. These display vertical streaks of donor-derived melanophores spanning the entire flank from dorsal to ventral, including the fins, enlarged in I′,J′ J′′. These streaks appeared in the same rostrocaudal position as the larval melanophores. |
|
The Role of ErbB signalling and the DRGs for adult melanophore pigmentation. (A) hps/erbb3bt21411 adult fish showing a dramatic regional reduction of melanophores. (B-E) Adult fish treated at successive stages of somitogenesis (left) with the ErbB inhibitor PD168393. The later the inhibitor was applied, the more posterior is the position of the defect (brackets). (F) Adult fish in which eight consecutive DRGs were laser-ablated at 3 dpf. PHENOTYPE:
|
|
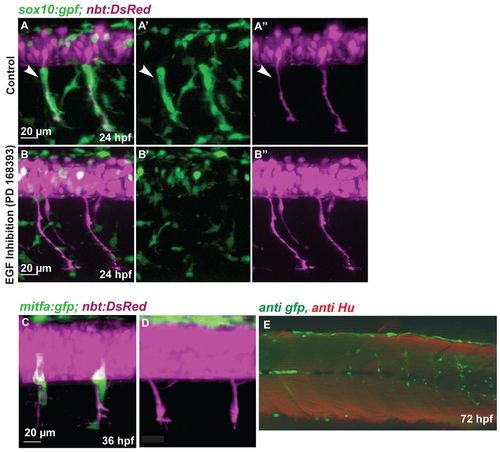
NC migration along the ventromedial path is blocked by inhibition of ErbB receptors. (A-B′′) Tg(sox10:gfp; nbt:DsRed) zebrafish embryos at 24 hpf. (A,B) Red and green channel (merge); (A′,B′) green channel; (A′′,B′′) red channel. Medial NC cells (green) covering the primary motor axons (white arrowheads, A-A′′) are absent after treatment with the ErbB inhibitor PD168393 (B-B′′). (C,D) Confocal images of 36 hpf Tg(mitfa:gfp; nbt:DsRed) embryos. (C) Wild-type embryo. (D) Embryo treated with ErbB inhibitor at 16 hpf. (E) Tg(mitfa:gfp) embryos were injected with a double MO combination against mitfa and erbb3b. Larvae were stained at 8 dpf using anti-GFP (green) and anti-HU (red) antibody (white arrowheads). The association of DRGs (red) and GFP-positive cells was quantified (Table 1). In double mitfa and erbb3b knockdowns, of 82 metamers lacking HU positive cells only five (6%) develop a string of GFP-positive cells. |
|
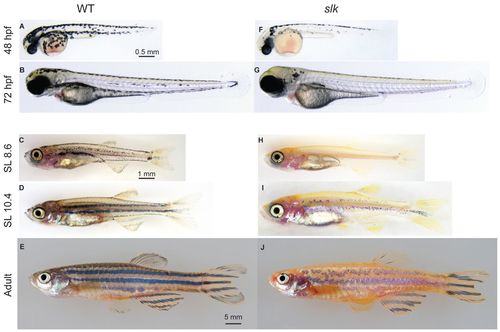
The phenotype of sparse liketc244b fish at different developmental stages. (A-J) The phenotype of wild-type (A-E) and sparse like (F-J) fish at different developmental stages. Unlike wild-type fish (C), slk fish are almost completely devoid of melanophores at the beginning of metamorphosis (H). Adult slk mutants (J) form stripes with strong reduction in melanophore number compared with wild-type siblings (E). SL, standard length. |
|
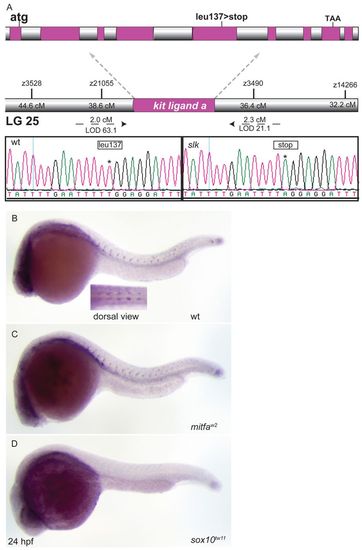
slk encodes Kitlga and is expressed in NC cells. (A) slk mutants reveal a T to A base substitution in exon 5 of the kitlga transcript causing a premature stop codon. (B-D) kitlga in situ hybridisation. At 24 hpf, kitlga is expressed in NC cells migrating along the medial path. This expression domain is maintained in mitfa mutants (C) but not in sox10 mutant embryos (D). EXPRESSION / LABELING:
|
|
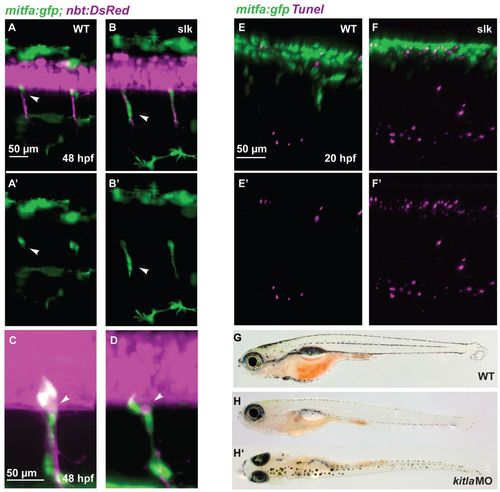
Abnormal NC migration and apoptosis in slk embryos. (A-B′) GFP-positive cells in double transgenic Tg(mitfa:gfp; nbt:DsRed) wild-type (A,A′) and slk mutant (B,B′) embryos at 48 hpf. In the wild-type embryo, GFP-positive NC cells remain at the position of the DRG and have a rounded morphology (white arrowhead in A,A2) whereas in slk mutants they are stretched along the nerves and appear to be migratory (white arrowheads in B,B2). A′, B′ show green channel only. (C,D) Magnification showing mitfa:gfp-labelled cells at the exit point of the spinal nerves at 48 hpf of wild-type (C) and slk mutant (D) embryos. The arrowheads point to DRGs. (E-F′) TUNEL staining in Tg(mitfa:gfp) embryos in wild-type (E,E′) and slk (F,F′) embryos at 20 hpf. E′,F′ show red channel only. (G-H′) Wild type (G) and slk morphant (H,H′) at 20 dpf (5.7 mm SL). EXPRESSION / LABELING:
PHENOTYPE:
|

Melanoblast migration along the ventromedial path is blocked in erbb2b mutants. Imaging of Tg(mitfa:gfp; nbt:DsRed) embryos between 20 and 30 hpf, 1 frame per 15 minutes. The motoneurons remain uncovered by GFP-positive cells, whereas some melanoblasts can be seen migrating ventrally along the dorsolateral path. Movie 1 shows the wild type for comparison. PHENOTYPE:
|