- Title
-
WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates KCC2 Expression and Posterior Lateral Line Development in Zebrafish (Danio rerio)
- Authors
- Bercier, V., Brustein, E., Liao, M., Dion, P.A., Lafrenière, R.G., Rouleau, G.A., and Drapeau, P.
- Source
- Full text @ PLoS Genet.
|
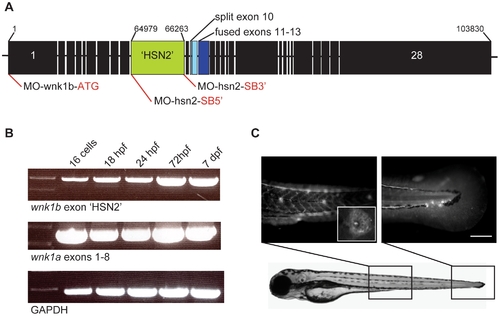
Expression of the WNK1 kinase in zebrafish embryos. A) Structure of the zebrafish WNK1 ortholog which conserved the HSN2 exon, wnk1b. The split exon 10 and fused exons 11–13 have been indicated on the sequence, respectively in pale blue and dark blue, as well as antisense morpholino oligonucleotide targets (red lines). B) Both copies of the zebrafish WNK1 ortholog, wnk1a and wnk1b are expressed early at the 16 cells stage and persist at 18, 24 and 72hours post-fertilization (hpf) and until 7 days post-fertilization (dpf). The RT-PCR was done using primers set in the HSN2 exon for wnk1b, targeting a sequence spanning exons 1–8 for wnk1a and using as control the housekeeping gene GAPDH. C) Zebrafish WNK1/HSN2 from the wnk1b gene was detected in the neuromasts of the posterior lateral line by whole-mount immunohistochemistry using an anti-HSN2 antibody. The inset shows a closer view of a stained neuromast. Scale bar: 100μm. |
|
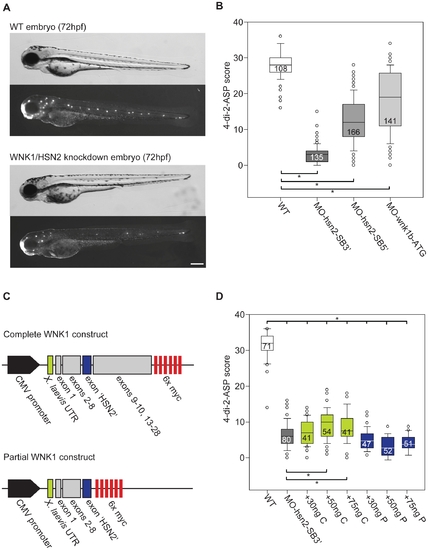
WNK1/HSN2 knockdown in zebrafish using antisense morpholino oligonucleotides (AMO). A) Knockdown embryos show no morphological phenotype, but reveal posterior lateral line defects (PLL) as observed under fluorescence with the 4-di-2-ASP vital dye when compared with non-injected WT embryos at 72hours post-fertilization. The knockdown embryo presented is a representative result obtained from MO-hsn2-SB52 injection. B) Each neuromast of the PLL observed with 4-di-2-ASP is assigned a score and totals for each fish is tabulated by condition and presented as a box plot, showing significantly lower scores for knockdown embryos when compared with WT. C) Two human sequence constructs of WNK1/HSN2 were designed for the rescue of the knockdown phenotype: a partial sequence containing exons 1-HSN2 and a complete construct containing exons 1–28 (but missing exons 11 and 12). D) 4-di-2-ASP score was assessed for embryos injected with MO-hsn2-SB32 and concentrations of 30, 50 or 75ng/μl of either partial or complete human WNK1/HSN2 constructs, revealing a partial rescue of the knockdown phenotype for embryos injected with 50 and 75ng/μl of the complete construct. Scale bar: 100μm. PHENOTYPE:
|
|
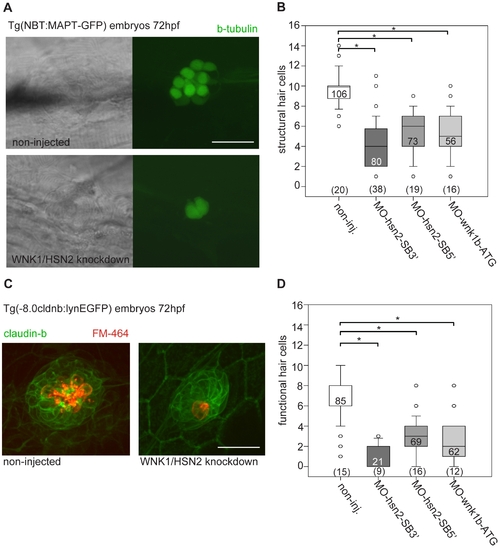
WNK1/HSN2 knockdown leads to abnormal neuromast development. A) The number of structural hair cells was assessed using transgenic embryos expressing GFP under the beta-tubulin promoter, revealing only the neuronal hair cells within PLL neuromasts. C) The number of functional hair cells was assessed by using transgenic embryos expressing GFP under the claudin-b promoter, rendering the neuromast fluorescent. The functional hair cells were revealed by incubation in the styryl dye FM-464FX, shown in red. B, D) Hair cells were counted for each PLL neuromast and totals were tabulated in box plots showing that WNK1/HSN2 knockdown embryos have a significantly lower number of structural and functional hair cells within their neuromasts when compared with non-injected embryos. The knockdown embryos presented in (A) and (C) are representative results at 72hpf obtained from MO-hsn2-SB32 injection. The number of neuromasts counted per condition is indicated in the boxes and the total number of embryos obtained per condition is indicated in parenthesis at the bottom of the box plots. Scale bar: 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
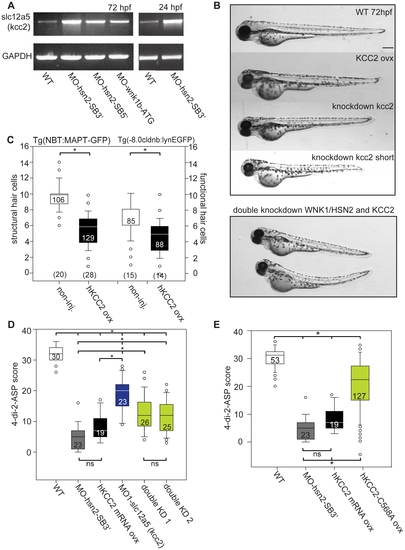
KCC2 is overexpressed in WNK1/HSN2 knockdown embryos. A) RT-PCR against slc12a5 (coding for kcc2) shows higher levels of RNA in WNK1/HSN2 knockdown embryos when compared with WT at 72hpf as well as at 24hpf. We overexpressed human KCC2 mRNA in WT embryos (morphology presented in (B)) to validate this result and were able to replicate the WNK1/HSN2 knockdown phenotype as assayed by (C) the number of structural and functional hair cells in PLL neuromasts and by (D) the 4-di-2-ASP score. We also validated this result by obtaining a partial rescue of the WNK1/HSN2 knockdown phenotype by knocking down kcc2 using MO1-slc12a5 in WNK1/HSN2 embryos (double knockdown-KD experiments). The embryos lacking kcc2 have morphological defects and a lower 4-di-2-ASP score (D) due to their smaller length (B), but double knockdown embryos, which are also morphologically abnormal and smaller in size have a significantly higher 4-di-2-ASP score (D) indicative of a partial rescue. For (C) The number of neuromasts counted per condition is indicated in the boxes and the total number of embryos obtained per condition is indicated in parenthesis at the bottom of the box plots. For (D), the total number of embryos is indicated in the boxes. (E) Overexpression of inactive mutant KCC2-C568A mimics hKCC2 overexpression and WNK1/HSN2 knockdown phenotype by producing PLL defects as assayed by 4-di-2-ASP vital dye staining. This indicates that WNK1/HSN2 interacts with KCC2 and regulates its transcription independent of the cotransporter′s activation. Neuromast scores were tabulated as previously done and presented as a box plot. The number of neuromasts counted per condition is indicated in the boxes. Scale bar: 100μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
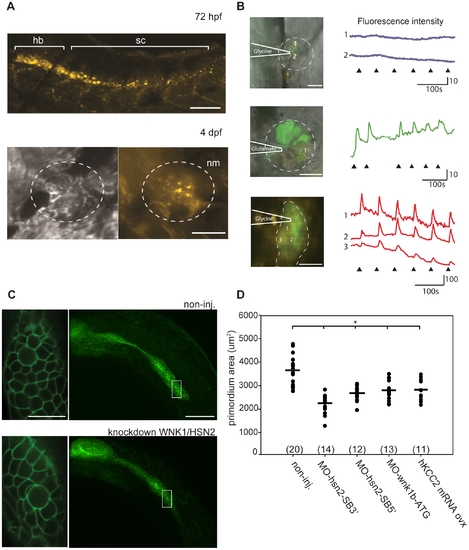
KCC2 is found in the embryonic zebrafish PLL. A) Presence of kcc2 in the zebrafish embryo was assessed by in situ hybridization against slc12a5 and reveals staining in the hindbrain (hb) and spinal cord (sc) at 72hpf as well as staining in hair cells of a PLL neuromast (nm) at 4dpf. B) Neuromasts of 4 dpf transgenic Tg(NBT:MAPT-GFP) embryos expressing GFP (green) under neuron-specific beta-tubulin promoter (left upper and middle images) and primordium of a 2dpf transgenic Tg(-8.0cldnb:lynEGFP) embryos expressing GFP under the claudin-b promoter in the membranes of cells composing the primordium (left lower image) were labeled with the Ca2+ indicator Rhod 2-AM (deep red) to show that ionophoresis of glycine failed to evoke Ca2+ transients in 3–4 dpf neuromasts (right black upper traces) but did so in primordium cells (right red bottom traces). In contrast, neuromasts of embryos of equivalent stage respond to glutamate (right, green middle trace). The dashed line illustrates the primordium or neuromast region and the heavy lines illustrates the position of the pipet. C)Transgenic embryos expressing GFP under the claudin-b promoter, which labels the membranes of cells composing the primordium, were used to observe the size of this migrating group of PLL neuromast progenitors for both non-injected and WNK1/HSN2 knockdown embryos. Close-up image of the primordium cells shows no difference in organization between non-injected and WNK1/HSN2 knockdown embryos. D) The primordium area was measured on one side of the embryos and data was tabulated in a scatter plot which shows that WNK1/HSN2 embryos, as well as embryos overexpressing human KCC2, have a significantly smaller primordium area than non-injected embryos. The number of primordial measured is indicated in parenthesis at the bottom of the graph. The knockdown embryo presented in (C) is a representative results at 22hpf obtained from MO-wnk1-ATG injection. Scale bars: (A) 100μm and 20μm for neuromast image, (B)20μm, (C) 80μm for full primordium and 20μm for cell close-up. EXPRESSION / LABELING:
PHENOTYPE:
|
|
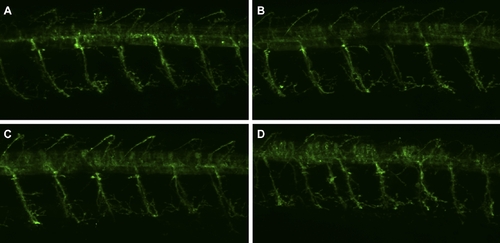
Motor neurons of wnk1/hsn2 knockdown embryos are not morphologically abnormal. Wnk1/hsn2 knockdown embryos expressing GFP under the HB9 promoter (Tg(mnx1:GFP) from Flanagan-Street et al., 2005) were obtained for all three AMOs conditions (A-non injected embryo, B- MO-wnk1-ATG, C- MO-hsn2-SB52, D- MO-hsn2-SB32). The primary motor neurons did not show any defects at 48hpf, which further parallels the HSANII pathology, where patients have no motor dysfunction. |