- Title
-
ENC1-like Integrates the Retinoic Acid/FGF Signaling Pathways to Modulate Ciliogenesis of Kupffer's Vesicle during Zebrafish Embryonic Development
- Authors
- Qian, M., Yao, S., Jing, L., He, J., Xiao, C., Zhang, T., Meng, W., Zhu, H., Xu, H., and Mo, X.
- Source
- Full text @ Dev. Biol.
|
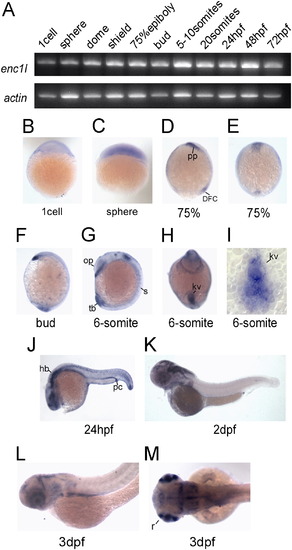
Enc1l expression pattern analysis in zebrafish. (A) RT-PCR reveals that enc1l is expressed throughout embryonic developmental stages. (B–M) In situ hybridization shows the expression pattern of enc1l during embryonic development. Images of (B) the 1-cell stage and (C) sphere stage show that enc1l is ubiquitously distributed before the gastrula stage. (D, E): In 75% epiboly, enc1l is expressed in the prechordal plate and dorsal forerunner cells. (F): enc1l is expressed in the prechordal plate and the tail bud in the bud stage. (G–I): enc1l is expressed in the 6-somites stage. (G): enc1l is expressed in developing somites, otic primordium, and the tail bud. (H): A ventral view shows that enc1l is expressed in the Kupffer’s vesicle (KV). (I): A dorsal view of a flat-mounted tailbud shows that enc1l is expressed in the KV. (J): In 24hpf, enc1l is mainly expressed in the hindbrain, spinal cord, optic vesicles, and pronephric duct. (K): In 2dpf, enc1l is mainly expressed in the brain and retina. (L, M): enc1l is expressed in retina, brain, and endodermal organs at 3dpf. pp: prechordal plate; kv: Kupffer’s vesicle; DFC: dorsal forerunner cells; s: somites; tb: tail bud; op: otic primordium; pc: pronephric duct; hb: hind brain; r: retina; sc: spinal cord. EXPRESSION / LABELING:
|
|
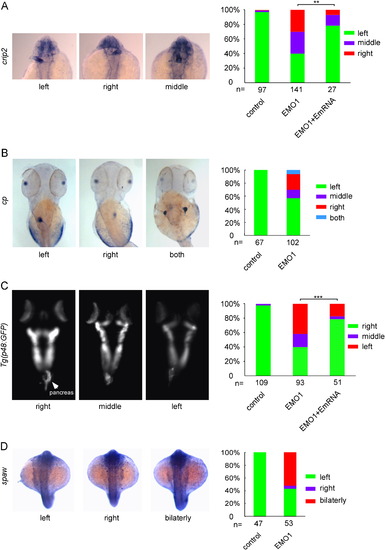
Enc1l is required for zebrafish left-side gene spaw and organs LR asymmetric formation. (A) Expression of crip2, a marker for heart, as well as the statistical graph indicate that 40%, 30%, and 30% of enc1l-MO1 embryos show that the heart position is on the left-side, middle, and right-side, respectively. The phonotype caused by knockdown of enc1l can be rescued by enc1-mRNA. (B): The liver marker cp and the statistical graph show that disruption of the liver in enc1l morphants cause 57.4%, 13%, 24.1%, and 5.5% of the morphants to have the liver positioned on the left, middle, right, and both positions, respectively. (C): P48:GFP transgenic fish and the statistical graph show that 42.4%, 18.1%, and 39.5% of the morphants have left, middle, and right-side pancreas, respectively. The phonotype caused by knockdown of enc1l can be rescued by enc1-mRNA. (D) WISH and the statistical graph show that approximately 53% of the morphants have bilateral and 4% of the morphants have right-side expression of spaw. **:0.005<p<0.01; ***: p<0.005. EXPRESSION / LABELING:
PHENOTYPE:
|
|
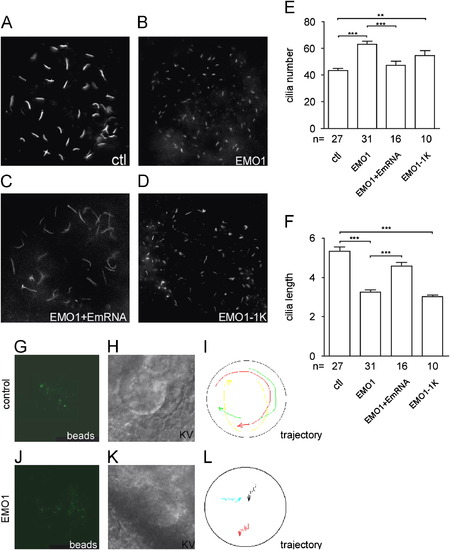
Enc1l is essential for KV ciliogenesis and KV function. (A–F) Anti-acetylated tubulin antibody is used to visualize the cilia in KV. (A) Control embryos, (B) enc1l-MO1 is injected at 1-cell stage, (C) enc1l-MO1 and enc1l-mRNA are co-injected at 1-cell stage. (D) enc1l-MO1 is injected at the 1000-cell stage (EMO1-1K). (E, F) Statistical graphs show the decrease in cilium length and increase in cilium number. (G–L): Enc1l is required for counterclockwise nodal fluid flow. (G–I) Still images from control-MO embryos and (J–L) enc1l-MO injected embryos. These images are taken from supplement Movie1 and 2, respectively. (G, J): Fluorescent beads in the KV. (H, K): The KV structure from the DIC image. (I, L): Trajectory of the fluorescent beads in KV. **0.005<π<0.01; *** :p<0.005. EXPRESSION / LABELING:
PHENOTYPE:
|
|
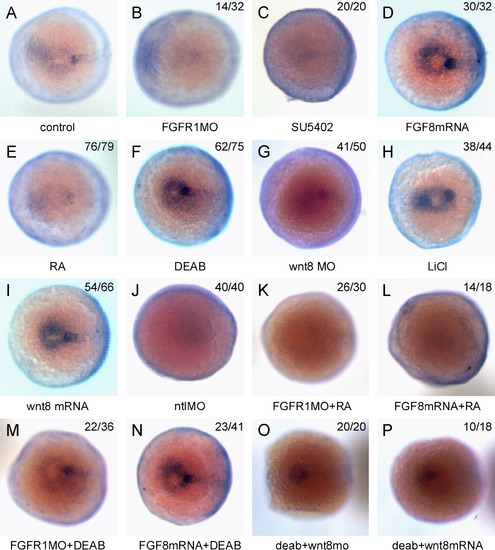
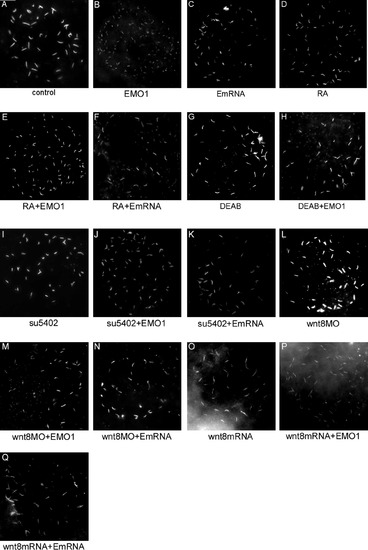
Enc1l expression is controlled by RA, FGF, and Wnt signaling in the KV of zebrafish embryos. The embryos were stained at the bud stage. (A): In control embryos, enc1l is expressed in the KV. (B, C) In fgfr1-MO injected or SU5402-treated embryos, the expression of enc1l in KV is reduced compared to control. (D): The enc1l expression level in KV is increased by over-expression of fgf8-mRNA. (E): The enc1l expression level is reduced in RA-treated embryos. (F): The enc1l expression level is increased by DEAB treatment. (G): Injection of wnt8-MO reduces enc1l expression in the KV. (H): Treatment with LiCl, which increases canonical Wnt signaling, increases enc1l expression level. (I): Overexpression of wnt8-mRNA increases enc1l expression. (J): Injection of ntl-MO can reduce the enc1l expression level. Up- (L) and down- (K) regulation of FGF signaling has no effect on RA-treated embryos; however, in DEAB-treated embryos, the down-regulation of FGF signaling (M) can restore the enc1l expression level, though the up-regulation of FGF signaling (N) has no affect. In DEAB-treated embryos, injection of wnt8-MO (O) or wnt8-mRNA (P) restores enc1l expression. The number of the representative phenotype embryos versus the number of total analyzed embryosis shown at the top of the right-hand corner of the picture. EXPRESSION / LABELING:
|
|
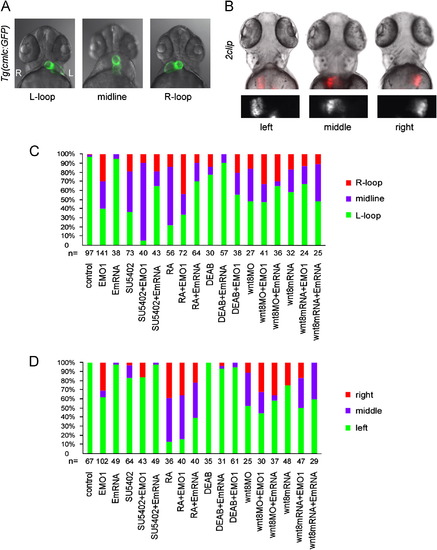
Organ LR asymmetric pattern shows that Enc1l functions as a downstream effector of RA and FGF signaling. (A) Knockdown of Encl1 shows L-loop, midline, and R-loop heart at 52phf. The embryos treated with SU5402 or RA also show disrupted LR asymmetry of the heart, which can be rescued by injected with encl1 mRNA. (B) The embryos treated with SU5402 or RA show disrupted LR asymmetry of liver at 72hpf, which can be rescued by injection with encl1 mRNA. The second line is the fluorescence images that only shows the liver LR asymmetric pattern. (C) Statistical graph of the heart location and (D) Statistical graph of the liver location. L: left side; R: right side. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Enc1l is conserved between zebrafish, mouse, and human.(A) The amino acid sequence alignment and (B) homology tree among zebrafish, mouse, and human ENC1 and zebrafish Enc1l show that Enc1l is highly conserved across vertebrate species. |
|
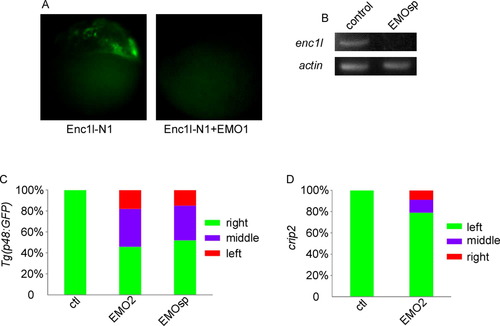
Enc1l-MO2 and enc1l-MOsp can affect LR organ asymmetry.(A): enc1l-MO1 efficiently blocks the expression of Enc1l-GFP fusion constructs injected into zebrafish embryos at the 1-cell stage. (B): RT-PCR shows that enc1l-MOsp causes abnormal reduction of enc1l-mRNA but not the internal β-actin mRNA control. (C): Injection of enc1l-MO2 or enc1l-MOsp disrupts the pancreas LR asymmetry. (D): Injection of enc1l-MO2 disrupts the heart LR asymmetry. |
|
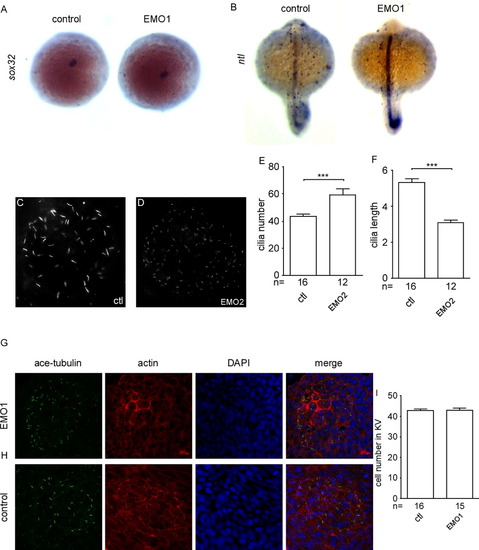
Knockdown of enc1l does not affect the midline, KV structure formation or KV cell number. (A): There is no difference in the KV marker sox32 between enc1l-MO injected embryos and control embryos. (B) No difference in the midline marker ntl is detected between enc1l morphants and control embryos. Injected enc1l-MO2 (D) can decrease the cilia length and increase the cilia number compared to control (C) embryos. (E): Statistical graph of the cilia number. (F): Statistical graph of the cilia length. Enc1l-MO1 (G) and control (H) embryos stained with ace-tubulin (green), phalloidine (red), and DAPI (blue). (I): Statistical graph of the KV cell number. ***: p<0.005. |
|
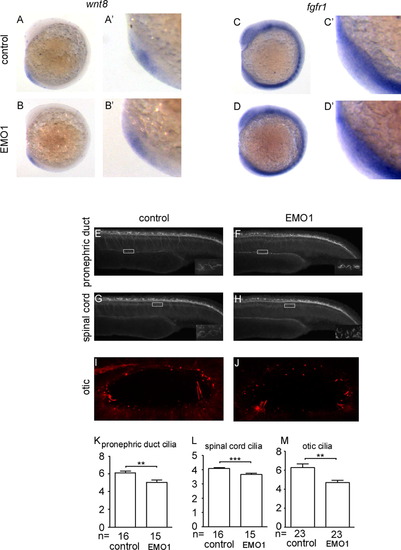
Wnt8 and fgfr1 expression levels are not affected by knockdown enc1l. Enc1l-MO1 affects the cilia of spinal cord, otic, and pronephric ducts.(A–B′) Wnt8 expression level is not affected by enc1l-MO1. (A): control embryos. (B): enc1l-MO injected embryos. (A′, B′): Magnified images of the KV structure. (C–D′) Fgfr1 expression level is not affected by enc1l-MO1. (C): control embryos. (D): enc1l-MO injected embryos. (C′, D′): Magnified images of the KV structure. (E–M): enc1l-MO1 affects the cilia length in the spinal cord, otic vesicles, and pronephric ducts. (E, G, and I) control embryos. (F, H, and J) enc1l-MO injected embryos. The magnified images of the white boxes are on the right bottom of the images. (K–M): Statistical graph of the cilia length of pronephric ducts (K), spinal cord (L), and otic vesicles (M). **: 0.005 <p<0.01; ***: p<0.005. |
Reprinted from Developmental Biology, 374(1), Qian, M., Yao, S., Jing, L., He, J., Xiao, C., Zhang, T., Meng, W., Zhu, H., Xu, H., and Mo, X., ENC1-like Integrates the Retinoic Acid/FGF Signaling Pathways to Modulate Ciliogenesis of Kupffer's Vesicle during Zebrafish Embryonic Development, 85-95, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.