- Title
-
Teleost growth factor independence (gfi) genes differentially regulate successive waves of hematopoiesis
- Authors
- Cooney, J.D., Hildick-Smith, G.J., Shafizadeh, E., McBride, P.F., Carroll, K.J., Anderson, H., Shaw, G.C., Tamplin, O.J., Branco, D.S., Dalton, A.J., Shah, D.I., Wong, C., Gallagher, P.G., Zon, L.I., North, T.E., and Paw, B.H.
- Source
- Full text @ Dev. Biol.
|
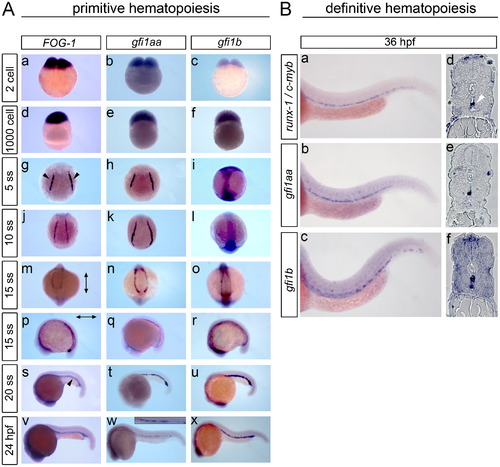
gfi1aa and gfi1b expression pattern during primitive and definitive hematopoiesis. The expression pattern of gfi1aa and gfi1b was detected by WISH of developing zebrafish embryos. (A) For the primitive wave of hematopoiesis, FOG-1 was included as a reference control. Dark-blue staining indicates pre-zygotic maternal expression of gfi1aa and gfi1b transcripts, along with FOG-1, at the 2-cell and 1000-cell stages (a–f). At the 5 and 10 ss, zygotic gfi1aa and FOG-1 expression is localized to the LPM (black arrowheads) (g–h, j–k), while gfi1b is expressed in the ectoderm (i, l). As the LPM converges to the ICM at the 15 ss, gfi1aa and FOG-1 expression remains localized to the LPM (j–k, m, n), while gfi1b transitions from ectoderm to LPM expression (l, o). (The vertical arrow indicates the anterior–posterior axis and the horizontal arrow indicates the rostral–caudal axis.) gfi1aa and gfi1b are strongly expressed in the ICM at the 20 ss along with FOG-1 (s–u). At 24 hpf, gfi1b and FOG-1 remain strongly expressed in the ICM, while expression of gfi1aa shows a relative decrease in ICM expression (v–x, insert). (B) AGM expression of gfi1aa and gfi1b during definitive hematopoiesis was analyzed by whole-mount and sectioned in situs using runx-1 and c-myb as reference controls (a–f). The white arrowhead indicates AGM expression of runx-1 and c-myb in the ventral wall of the dorsal aorta (d). |
|
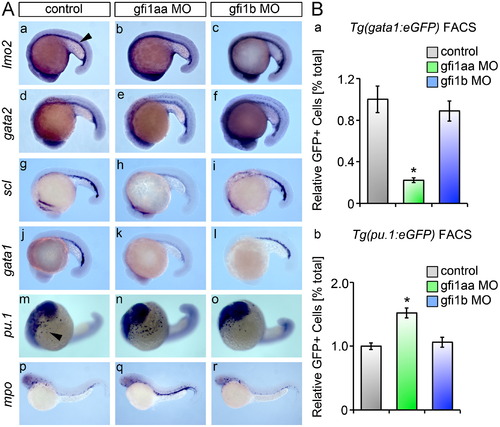
gfi1aa regulates the primitive wave of zebrafish hematopoiesis. (A) gfi morphants and matching controls were subjected to WISH and analyzed for ICM expression (arrowhead) of primitive hematopoietic markers at the 20 ss. (a–f) ICM expression of the early hematopoietic markers, lmo2 and gata2, is preserved in gfi morphants and controls. (g–l) gfi1aa morphants show a reduction in ICM expression of scl and gata1, markers of primitive hematopoietic progenitors as compared to controls. Loss of gfi1b does not affect scl or gata1 expression in primitive hematopoiesis (c, f, i, l). (m–o) Loss of gfi1aa shows an expansion in the population of pu.1 myeloid progenitors, while no change is observed in gfi1b morphants. (p–r) The mpo myeloid population is expanded in gfi1aa morphants. (B) Transgenic reporter fish for primitive wave hematopoietic progenitors were subjected to FACS analysis after morpholino-mediated knockdown. (a) FACS of Tg(gata1:eGFP) embryos at the 20 ss reveals a significant decrease in the population of erythroid progenitors in gfi1aa morphants relative to controls. Erythroid progenitors are unaffected in gfi1b morphants (mean±SE, t test, □p<0.05, n=3). (b) FACS of Tg (pu.1:eGFP) embryos at the 20 ss reveals a significant increase in the population of myeloid progenitors in gfi1aa morphants relative to controls. Myeloid progenitors are unaffected in gfi1b morphants (mean±SE, t test, □p<0.05, n=5). PHENOTYPE:
|
|
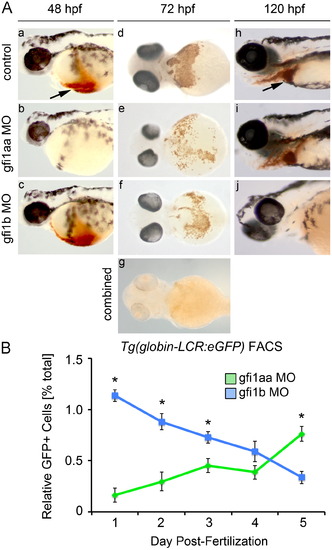
gfi1aaandgfi1b have distinct roles at different stages in erythropoiesis. (A) Morphant and control embryos were stained with o-dianisidine to detect hemoglobinized cells. At 48 hpf, knockdown of gfi1aa results in severe anemia, as indicated by the absence of hemoglobinized cells in the cardiac region (arrow) (a-b). Knockdown of gfi1b does not impact hemoglobinization at this stage (c). At 72 hpf, knockdown of either gfi1aa or gfi1b partially reduces hemoglobinized erythrocytes relative to control embryos, while knockdown of both genes results in a more profound reduction of hemoglobinized erythrocytes (d–g). At 120 hpf, gfi1aa morphants have recovered from their initial anemia, as indicated by the presence of hemoglobinized cells, whereas gfi1b morphants are severely anemic (h–j). (B) FACS of Tg(globin-LCR:eGFP) embryos injected with MO targeting either gfi1aa (green) or gfi1b (blue) quantifies the relative change in erythrocytes over time, showing that loss of either gfi1aa or gfi1b differentially impacts erythropoiesis at different stages of development (mean±SE, t test, □p<0.05, n=3). |
|
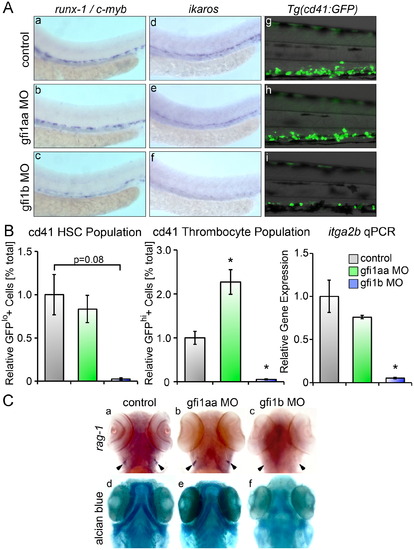
Loss ofgfi1b silences definitive HSC. (A) Loss of gfi1b silences runx-1, c-myb and ikaros expressing HSC in the AGM at 36 hpf relative to matched controls (a–f). Loss of gfi1b also reduces expression of GFP+cd41 cells in Tg(cd41:eGFP) embryos at 96 hpf (d–f). (B) FACS of Tg(cd41:eGFP) embryos at 96 hpf injected with MO targeting either gfi1aa or gfi1b reveals a marked reduction in the GFPlo and GFPhi populations in gfi1b morphants (mean±SE, t test, □p<0.05, n=3). qRT-PCR of gfi morphants shows significantly reduced expression of itga2b (cd41) in gfi1b morphants at 96 hpf (mean±SE, t test, *p<0.05, n=3). (C) Loss of gfi1b silences rag-1 expressing thymic lymphocytes, while loss of gfi1aa has no effect on lymphopoiesis in the thymic anlage (a–c). (d–f) Embryos were stained with Alcian Blue to delineate the morphologic architecture of the jaw cartilages. gfi1aa morphants have normal jaw cartilage development compared with wild type controls (d, e), while gfi1b morphants show dysplastic development of the jaw cartilage (f). |
|
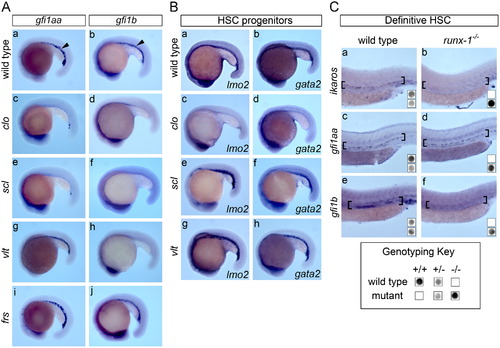
gfi1aa and gfi1b function at different hierarchical levels in hematopoietic epistasis. (A) The ICM expression of gfi1aa and gfi1b was evaluated in mutants with genetic blocks at sequential stages in hematopoiesis at the 20 ss. (a–b) Both gfi1aa and gfi1b are expressed in the ICM (arrowheads) in wild type embryos. (c–d) Mutants with genetic defects at the hemangioblast level (clo) and hematopoietic progenitors (scl) specifically lack ICM expression of either gfi1aa or gfi1b. Note that the neural expression of the gfi genes is preserved in these genetic mutants, confirming the specificity of the assay. (g–h) ICM expression of gfi1aa is preserved in the gata1 mutant, vlt, whereas gfi1b expression is deficient. (i–j) In contrast, both gfi1aa and gfi1b are expressed in the ICM of the mfrn1 mutant, frs. (B) To confirm the presence of hematopoietic progenitors in various genetic mutants, the expression of lmo2 and gata2 was evaluated in mutant embryos at the 20 ss. (a–b) Both lmo2 and gata2 are expressed in the ICM of wild type control embryos. (c–d) Neither lmo2 nor gata2 is expressed in the ICM of clo mutants, which fail to specify hematopoietic and vascular progenitors. (e–h) Expression of lmo2 and gata2 is preserved in scl and gata1 (vlt) mutants, confirming that hematopoietic progenitors are present. (C) The AGM expression of ikaros, gfi1aa and gfi1b was evaluated in runx-1 mutants at 36 hpf and embryos were genotyped for runx-1 wild type and mutant alleles. ikaros was included as a reference control. (a–b) AGM expression (brackets) of ikaros is silenced in runx-1 mutants relative to wild type embryos (wt), confirming the defect in definitive hematopoiesis. (c–d) AGM expression of gfi1aa is preserved in runx-1 mutants. (e–f) Loss of runx-1 silences expression of gfi1b in the AGM. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 373(2), Cooney, J.D., Hildick-Smith, G.J., Shafizadeh, E., McBride, P.F., Carroll, K.J., Anderson, H., Shaw, G.C., Tamplin, O.J., Branco, D.S., Dalton, A.J., Shah, D.I., Wong, C., Gallagher, P.G., Zon, L.I., North, T.E., and Paw, B.H., Teleost growth factor independence (gfi) genes differentially regulate successive waves of hematopoiesis, 431-441, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.