- Title
-
betaPix plays a dual role in cerebral vascular stability and angiogenesis, and interacts with integrin alphavbeta8
- Authors
- Liu, J., Zeng, L., Kennedy, R.M., Gruenig, N.M., and Childs, S.J.
- Source
- Full text @ Dev. Biol.
|
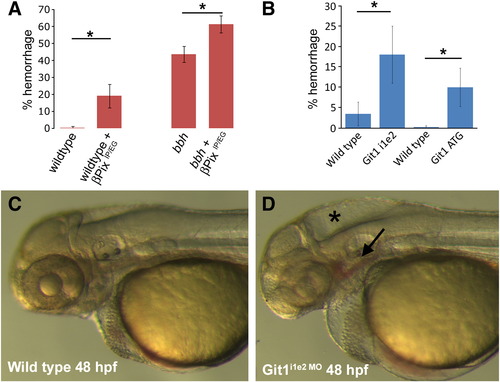
Mutation of Git1 binding residues in βPix or knockdown of Git1 leads to hemorrhage. A) Expression of mutant βPix lacking residues important for Git1 binding (βPix IP/EG) leads to increased hemorrhage in wild type vessels, and in bbhm292 mutants. B) Knockdown of Git1 by either a Git1i1e2 or Git1ATG morpholino leads to increased hemorrhage as compared to wild type embryos. C) Wild type embryo at 48 hpf. D) Git1i1e2 morphant shows hemorrhage (arrow) and hydrocephalus (star). PHENOTYPE:
|
|
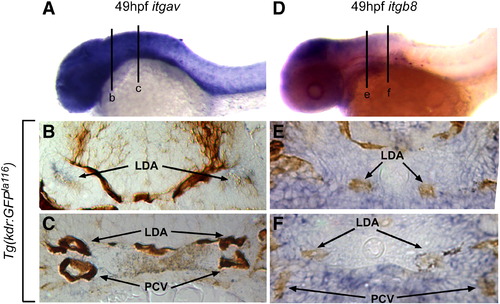
Integrin αv and β8 are expressed in close proximity to head blood vessels. Comparative in situ hybridization of integrin αv (itgav; blue staining in A–C) or integrin β8 (itgb8; blue staining in D–F) as assayed in Tg(kdrl:eGFP)la116 embryos marking endothelial cells (brown staining in B, C, E, F). (A) At 49 hpf itgav shows broad expression in the head and anterior ventral trunk. (B) In a transverse section of the head (line b in A), itgav is expressed in close proximity to the lateral dorsal aortae (LDA). (C) A more posterior transverse section (line c in A) shows itgav expression in the ventral mesenchyme of the head (VM), surrounding the lateral dorsal aortae (LDA) and posterior cardinal veins (PCV). (D) Similarly, at 49 hpf, itgb8 is highly expressed in the head and weakly expressed in the anterior ventral trunk. (E) In a transverse section of the head (line e in D), itgb8 is expressed in the regions around the lateral dorsal aortae (LDA). (F). A more posterior transverse section (line f in D) shows itgb8 expression in the ventral mesenchyme of the head (VM), surrounding the lateral dorsal aortae (LDA) and posterior cardinal veins (PCV). LDA: lateral dorsal aorta; PCV: posterior cardinal veins. EXPRESSION / LABELING:
|
|
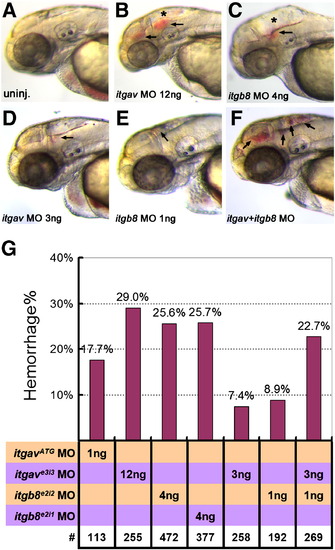
Loss of integrin αv, integrin β8 or combinatorial knockdown of both genes results in hemorrhage. As compared to uninjected controls in (A), 12 ng of itgave3i3 MO (B), or 4 ng of itgb8e2i2 MO (C), leads to head hemorrhages at 50–52 hpf. (D–F) Suboptimal morpholino doses (3 ng of itgave3i3 MO (D) or 1 ng of itgb8e2i2 (E)) results in small hemorrhages while double knockdown of both genes using these low doses results in numerous, larger hemorrhages (F, arrows). (G) Graphical representation of hemorrhage rate as a function of the dose of morpholino injected. Percentage of embryos with hemorrhages is shown above the bars. # represents the numbers of embryos scored for each analysis. PHENOTYPE:
|
|
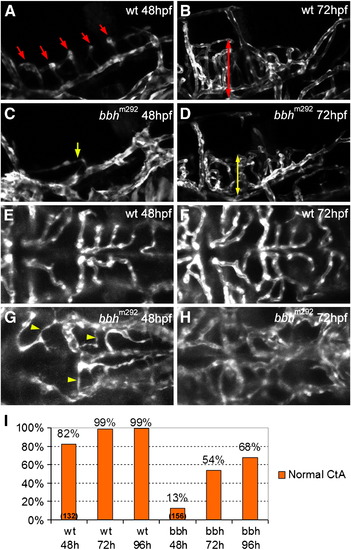
Defective hindbrain angiogenesis in bbhm292 mutants. (A) At 48 hpf, a lateral view of the hindbrain of the wild type embryo shows central arteries (CtA, red arrows) sprouting from posterior communicating segment (PCS) and basilar artery (BA) and draining into primordial hindbrain channel (PHBC) (B) At 72 hpf, the CtAs penetrate deeply into the hindbrain in wild type embryos (red double-headed arrow). (C) 48 hpf bbh homozygous mutants show a reduced number and atypical growth direction of the CtAs (yellow arrows). (D) At 72 hpf, the CtAs in bbh homozygotes invade into the hindbrain matter, but have reduced length as compared to wild type embryos (yellow double-headed arrow). (E) In a dorsal view regular, lumenized CtA are observed in 48 hpf wild type embryos. (F) At 72 hpf, the basic network structure of the CtA remains similar, although is more refined. (G) In contrast, 48 hpf bbh homozygotes show a reduced number of CtAs, with very irregular morphology (yellow arrowhead). (H) At 72 hpf, the structure of CtA vasculature in bbh homozygotes has an irregular morphology with abnormal connections and irregular vessel size. (I) Quantitation of the percentage normal CtAs at 48, 72 and 96 hpf in wild type embryos (wt) and bbh homozygotes (bbh). EXPRESSION / LABELING:
PHENOTYPE:
|
|
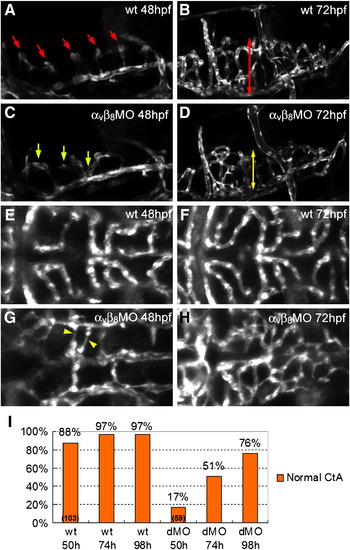
Defective hindbrain angiogenesis in integrin αv and β8 double morphants. (A) At 48 hpf, a lateral view of the hindbrain of the wild type embryo shows central arteries (CtA, red arrows) sprouting from posterior communicating segment (PCS) and basilar artery (BA) and draining into primordial hindbrain channel (PHBC) (B) At 72 hpf, the CtAs penetrate deeply into the hindbrain in wild type embryos (red double-headed arrow). (C) 48 hpf integrin αvβ8 double morphants show CtAs with reduced number and atypical growth direction (yellow arrows). (D) At 72 hpf, the CtAs in integrin αvβ8 morphants invade into the hindbrain matter, but have reduced length as compared to wild type embryos (yellow double-headed arrow). (E) In a dorsal view regular, lumenized CtA are observed in 48 hpf wild type embryos. (F) At 72 hpf, the basic network structure of the CtA remains similar, although is more refined. (G) In contrast, in 48 hpf integrin αvβ8 double morphants there is a reduced number of CtAs, with very irregular morphology (yellow arrowhead). (H) At 72 hpf, the structure of CtA vasculature in αvβ8 double morphants has an irregular, plexus-like morphology. (I) Quantitation of the percentage normal CtAs at 50, 74 and 98 hpf in wild type embryos (wt) and integrin αvβ8 double morphants (dMO). EXPRESSION / LABELING:
PHENOTYPE:
|
|
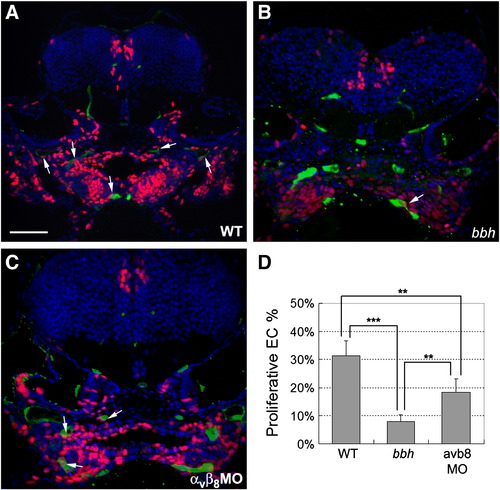
Defective endothelial cell proliferation in bbh mutants and integrin αvβ8 double morphants. (A–C) Cell proliferation was detected by pulsing with EdU at 48 hpf and fixation at 54 hpf (red), while endothelial cells are marked by expression of GFP in the Tg(kdrl:eGFP)la116 line (green) and nuclei are stained with DAPI (blue). In general, higher levels of EdU incorporation are detected in wild type embryos (A) as compared to bbh homozygotes (B) or and integrin αvβ8 double morphants (C). Arrows highlight proliferative endothelial cells. (D) The percentage of proliferative endothelial cells were analyzed from the percentage of cells with co-staining of EdU and GFP vs. the total number of GFP cells as observed on sections from 5 wild types, 5 bbh homozygotes, and 5 integrin αvβ8 double morphants. Significance was determined by a Student′s t test, where ** represents p < 0.005, and *** represents p < 0.001. |
|
Verification of transgene expression (A) Mosaic expression of βactin-βPixAIE-PGmyc detected at 52 hpf by immunofluorescence using antimyc staining shows a nontissue restricted mosaic expression in the whole fish. |
|
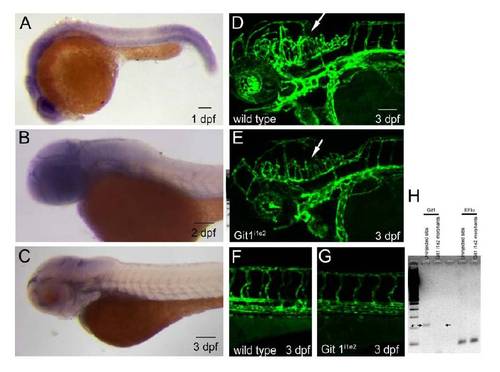
Git1 mRNA expression pattern and knockdown vascular phenotype (AC) In situ hybridization with a RNA probe against Git1 reveals ubiquitous staining at 1 day post fertilization (dpf) and 2 dpf. At 3 dpf, staining is mostly restricted to the head, but is still broadly distributed. (DF) Confocal microscopy of wild type head (D) and trunk (F) or Git1i1e2 morphant head (E) or trunk (G) at 3 dpf shows a largely normal vascular pattern in Tg(kdrla:GFP)la116 embryos. As compared to wild type embryos, the head vasculature in the region of the central arteries (CtAs) is underdeveloped (arrow). (H) Amplification of Git1 with primers surrounding the deleted exon shows that Git1 mRNA is absent in Git1i1e2 morphants, while the control gene EF1α amplifies normally. Scale bar in all panels represents 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
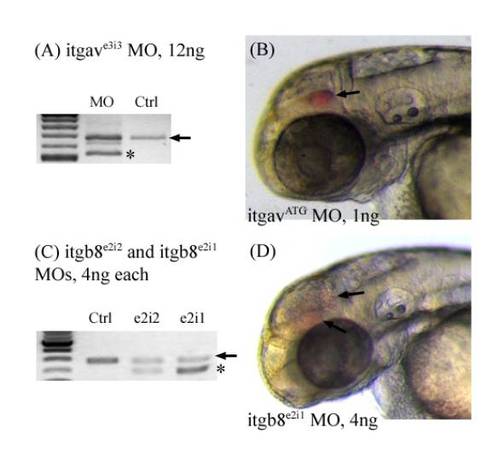
Morpholino efficiency test and hemorrhage phenotype for integrin morphants. (A) itgavMOf and itgavMOr primers were used to amplify integrin α v around the exonintron boundary targeted by the itgave3i3 MO. Black arrows denote the wildtype band. Stars denote misspliced new products seen only in the morphants. (B) Hemorrhages (arrow) are observed in the head of itgavATG morphants between 50 – 52 hpf. (C) itgb8MOf and itgb8MOr primers were used to amplify integrin β 8 around the exonintron boundary targeted by both itgb8e2i2 and itgb8e2i1 morpholinos. Black arrows denote the wildtype band, while a star denotes misspliced new products seen only in the morphants. (D) Hemorrhages (arrow) are observed in the head of itgb8e2i1 morphants. PHENOTYPE:
|
|
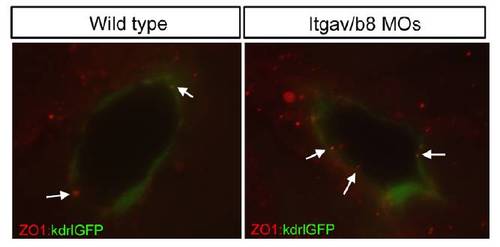
Distribution of ZO1 is unchanged in integrinαvβ8 morphants Staining with an antibody to the tight junction marker ZO1 in Tg(kdrla:GFP)la116 embryos shows no obvious difference in the localization of ZO1 between wild type embryos and integrinαvβ8 morphants. |
|
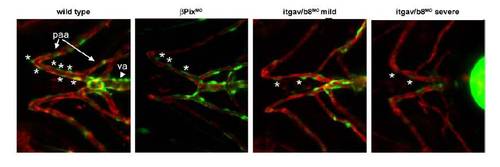
Reduction in mural cell coverage of ventral head vessels in βPix morphants and itgav/b8 double morphants Double transgenic Tg(αSMA:GFP)ca7; Tg(kdrla:mCherry)ci5 zebrafish expressing GFP under the mural cell αSMA promoter and mCherry under the endothelial kdrla promoter were injected with a morpholino to either βPix or morpholinos to itgav and itgb8 and assayed at 4 dpf for mural cell presence. Stars mark mural cells in an equivalent vessel in all panels. Abbreviations: va, ventral aorta; paa, pharyngeal arch artery. |
|
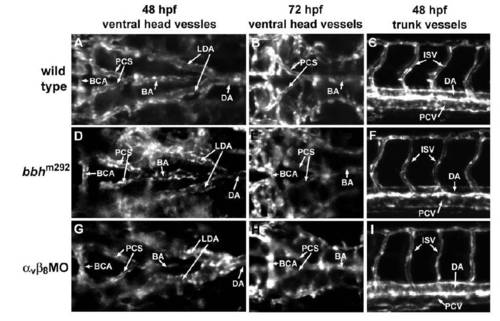
Early vascular pattern is normal in bbh mutants and α v β8 double morphants. Confocal images were taken in the ventral head at 48 hpf (A, D, G) and 72 hpf (B, E, H), and the lateral midtrunk at 48 hpf (C, F, I). The basic vascular pattern is similar in wild types (A, B, C), bbh mutants (D, E, F) and α v β8 double morphants (G, H, I). BCA: basal communicating artery; PCS: posterior communicating segment; BA: basilar artery; LDA: lateral dorsal aorta; DA: dorsal aorta; ISV: intersegmental vessel; PCV: posterior cardinal vein. EXPRESSION / LABELING:
|
|
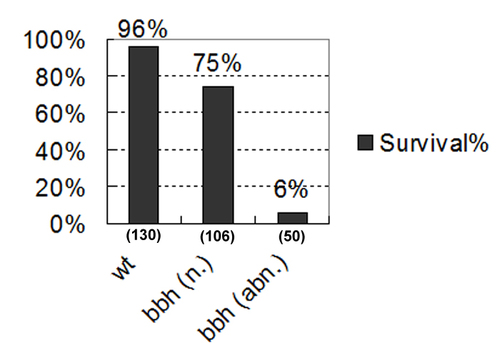
Correlation of reduced survival rate with abnormal CtA development in bbh homozygotes bbh homozygous mutants were scored for CtA morphology at 4 dpf and divided into two categories: normal CtA (n.) and abnormal CtA (abn.). The embryos were then were raised to 6 dpf. Healthy embryos with a normalsized swim bladder and normal morphology were scored as surviving. |
|
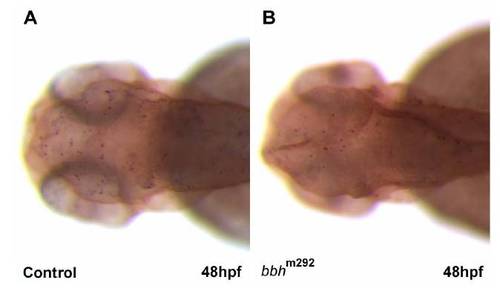
Cell death does not appear to contribute to the hemorrhage or angiogenic phenotype in bbh mutants TUNEL staining was performed to assess cell death in wild type controls (A) and bbh mutants (B) at 48 hpf. Similar patterns were observed. PHENOTYPE:
|
Reprinted from Developmental Biology, 363(1), Liu, J., Zeng, L., Kennedy, R.M., Gruenig, N.M., and Childs, S.J., betaPix plays a dual role in cerebral vascular stability and angiogenesis, and interacts with integrin alphavbeta8, 95-105, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.