- Title
-
The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain
- Authors
- Kizil, C., Dudczig, S., Kyritsis, N., Machate, A., Blaesche, J., Kroehne, V., and Brand, M.
- Source
- Full text @ Neural Dev.
|
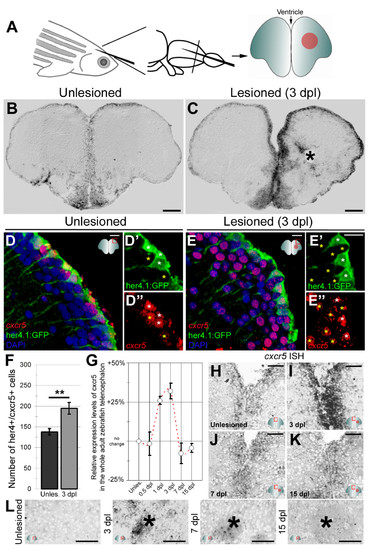
cxcr5 is expressed in radial glial cells (RGCs) and neurons in the adult zebrafish telencephalon. (A) Schematic representation of an adult zebrafish telencephalon. A stab lesion is performed in one hemisphere (red circle on the cross section scheme). (B)cxcr5 is expressed along the ventricular region in the unlesioned telencephalon. (C)cxcr5 expression after a lesion (asterisk) is stronger in the lesioned hemisphere along the ventricular region. (D)cxcr5 fluorescent in situ hybridization (FISH) coupled to green fluorescent protein (GFP) immunohistochemistry in Tg(her4.1:GFP) transgenics in unlesioned the adult zebrafish telencephalon; counterstained with 4,6-diamidino-2-phenylindole (DAPI). (D′) Individual channel for her4.1:GFP. (D′′) Individual channel for cxcr5. Radial glial cells (white asterisks) and periventricular cells (yellow asterisks) express cxcr5. (E)cxcr5 FISH coupled to GFP staining in Tg(her4.1:GFP) transgenics in the 3 day post-lesion adult zebrafish telencephalon; counterstained with DAPI. (E′) Individual channel for her4.1:GFP. (E′′) Individual channel for cxcr5. Radial glial cells (white asterisks) and periventricular cells (yellow asterisks) express cxcr5. Note the number of cxcr5-positive periventricular cells increased in comparison to the unlesioned region. (F) Graph indicating the number of her4-cxcr5 double-positive cells before and after inducing the lesion. (G) Quantitative real-time PCR analysis for cxcr5 expression at different time points after the lesion. (H-K) Time-course cxcr5 in situ hybridization analyses on the unlesioned region (H), 3 dpl (I), 7 dpl (J) and 15 dpl (K) telencephalons. (L)cxcr5 expression around the lesion site. Lesion site is denoted by an asterisk; n e 3 telencephalons for every analysis. Scale bars 50 μm (B, C, H-L), and 10 μm (D-E′′). EXPRESSION / LABELING:
|
|
cxcr5 is expressed in proliferating radial glial cells (RGCs). (A) Scheme for experimental setup. At 3 days after a lesion or a sham operation, bromo-deoxyuridine (BrdU) is applied for 6 hours before sacrificing the animals. (B) BrdU immunohistochemistry on the unlesioned (sham-operated) adult zebrafish telencephalon section showing the proliferating cells. (C)cxcr5 fluorescent in situ hybridization (FISH) coupled to BrdU and GFP immunohistochemistry on unlesioned Tg(her4.1:GFP) transgenics. Insets show single channel images. BrdU-positive RGCs express cxcr5 (white asterisk). (D) BrdU immunohistochemistry on the 3 days post-lesion (dpl) adult zebrafish telencephalon section. Asterisk indicates the lesion site. (E)cxcr5 FISH coupled to BrdU and GFP immunohistochemistry on 3 dpl Tg(her4.1:GFP) transgenics. Insets show single channel images. BrdU-positive RGCs increase in number and they express cxcr5 (white asterisks). Scale bars 50 μm in B and D, and 10 μm in C and E; n = 3 telencephalons. EXPRESSION / LABELING:
|
|
cxcr5 is sufficient to increase ventricular cell proliferation after injury in the adult zebrafish telencephalon. (A) Cxcr5 is a seven-span transmembrane protein with an extracellular receptor domain, seven transmembrane domains and a C-terminus intracellular domain. We generated full-length and dominant-negative versions of Cxcr5. Both variants are inserted into a transgenesis cassette containing the enhanced green fluorescent protein (EGFP) reporter and self-cleaving T2A. The whole cassette is expressed under heat-inducible hsp70l promoter. (B) Heat shock scheme. Four heat shocks, three of which are after the lesion or sham operation, were given before sacrifice and analysis. (C) Proliferating cell nuclear antigen (PCNA) and green fluorescent protein (GFP) immunohistochemistry (IHC) in telencephalons of unlesioned (sham-operated) non-transgenic animals. C′ and C′′ are PCNA and GFP. (D) PCNA and GFP IHC in telencephalons of unlesioned (sham-operated) Tg(hsp:egfp-t2a-dncxcr5) animals. D′ and D′′ are PCNA and GFP. (E) PCNA and GFP IHC in telencephalons of unlesioned (sham-operated) Tg(hsp:egfp-t2a-FLcxcr5) animals. E′ and E′′ are PCNA and GFP. (F) Quantification graph for the relative amounts of GFP and PCNA double-positive cells in unlesioned telencephalons, where the transgenic misexpression of cxcr5 does not effect cell proliferation. (G) PCNA and GFP IHC in telencephalons of 3 days post-lesion (dpl) non-transgenic animals. G′ and G′′ are PCNA and GFP. (H) PCNA and GFP IHC in telencephalons of 3 dpl Tg(hsp:egfp-t2a-dncxcr5) animals. H′ and H′′ are PCNA and GFP. (I) PCNA and GFP IHC in telencephalons of 3 dpl Tg(hsp:egfp-t2a-FLcxcr5) animals. I′ and I′′ are PCNA and GFP. (J) Quantification graph for the relative amounts of GFP and PCNA double-positive in 3 dpl telencephalons, where transgenic misexpression of the full-length cxcr5 increases but the dominant negative variant does not affect the ventricular cell proliferation. Scale bars 25 μm; n = 4 telencephalons for each set of analyses. |
|
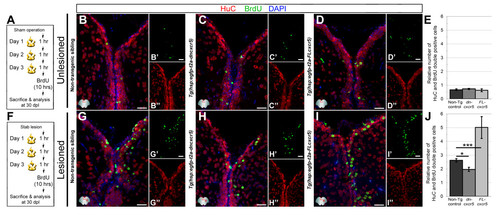
cxcr5 is required and sufficient for regenerative neurogenesis. (A) Heat shock and BrdU scheme for neurogenesis assay in the unlesioned telencephalon. After a sham operation, three daily heat shocks were given before a 10-hour BrdU pulse and sacrifice at 30 days after the sham operation. (B-D) HuC and BrdU immunohistochemistry (IHC) and 4,6-diamidino-2-phenylindole (DAPI) counterstaining on unlesioned telencephalons from non-transgenic (B), Tg(hsp:egfp-t2a-dncxcr5)(C) and Tg(hsp:egfp-t2a-FLcxcr5)(D) animals. Primed and double-primed images are single channels for BrdU and HuC. (E) Quantification graph for relative numbers of HuC and BrdU double positive cells (newborn neurons) in unlesioned telencephalons. Transgenic misexpression of cxcr5 does not alter the constitutive levels of neurogenesis in the adult zebrafish telencephalon. (F) Heat shock and BrdU scheme for neurogenesis assay in lesioned telencephalons. After the lesion, three daily heat shocks were given before a 10-hour BrdU pulse and sacrifice at 30 days after lesioning. (G-I) HuC and BrdU IHC and DAPI counterstaining on unlesioned telencephalons from non-transgenic (G), Tg(hsp:egfp-t2a-dncxcr5)(H) and Tg(hsp:egfp-t2a-FLcxcr5)(I) animals. Primed and double-primed images are single channels for BrdU and HuC. (J) Quantification graph for relative numbers of newborn neurons in lesioned telencephalons. Transgenic misexpression of dominant negative variant of cxcr5 significantly reduces, and full-length cxcr5 significantly increases, the number of newborn neurons after lesioning in the adult zebrafish telencephalon. Scale bars 25 μm; n = 4 telencephalons for each analysis. |
|
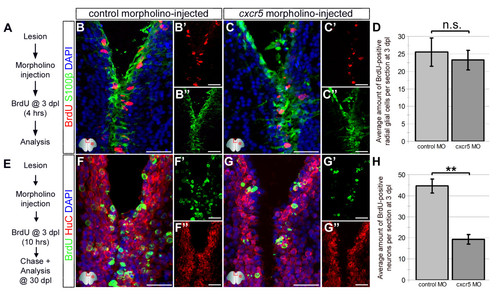
Knocking down cxcr5 with cerebroventricular microinjection (CVMI) results in reduced regenerative neurogenesis but not radial glial cell proliferation. (A) Lesion, CVMI and BrdU treatment scheme. (B) BrdU and S100β immunohistochemistry (IHC) with 4,6-diamidino-2-phenylindole (DAPI) counterstaining on control morpholino-injected brains. (B′) BrdU channel. (B′′) S100β channel. (C) BrdU and S100β IHC with DAPI counterstaining on cxcr5 antisense morpholino-injected brains. (C’) BrdU channel. (C′′) S100β channel. (D) Graph showing the average number of proliferating glial cells. Knocking down cxcr5 does not alter the levels of proliferating radial glial cells. (E) Lesion, CVMI, BrdU treatment and pulse-chase scheme. (F) BrdU and HuC IHC with DAPI counterstaining on control morpholino-injected brains. (F′) BrdU channel. (F′′) HuC channel. (G) BrdU and HuC IHC with DAPI counterstaining on cxcr5 antisense morpholino-injected brains. (G′) BrdU channel. (G′′) HuC channel. (H) Graph showing the average number of newborn neurons. Knocking down cxcr5 significantly reduces the levels of regenerative neurogenesis. |
|
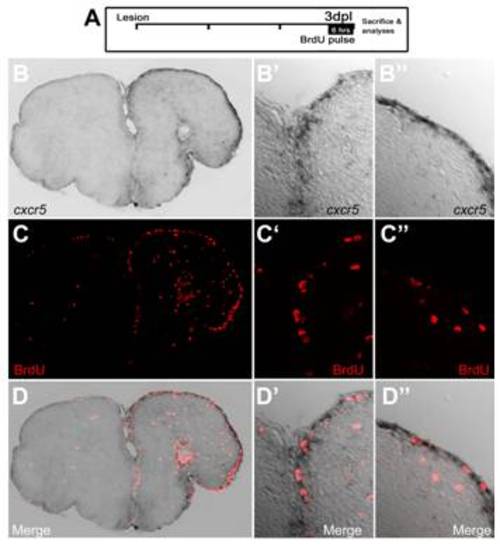
cxcr5 expression is overlapping to the proliferating ventricular cells in adult zebrafish telencephalon. (A) Lesion and bromo-deoxyuridine (BrdU) treatment paradigm. Fish were treated with BrdU 3 days post lesion (dpl) for 6 hours before the sacrifice. (B) cxcr5 in situ hybridization on lesioned telencephalon. (B′) High magnification of the dorsomedial region in (B). (B′′) High-magnification of the dorsolateral region in (B). (C) BrdU immunohistochemistry on lesioned telencephalon. (C′) High-magnification of the dorsomedial region in (C). (C′′) High-magnification of the dorsolateral region in (C). (D) Merged image of (B) and (C). (D′) Merged image of (B′) and (C′). (D′′) Merged image of (B′′) and (C′′). |
|
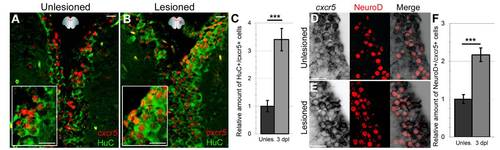
Neurons express cxcr5. (A) cxcr5 fluorescent in situ hybridization (FISH) coupled to HuC immunohistochemistry (IHC) on a section of unlesioned adult zebrafish telencephalon. Inset is magnified image. (B)cxcr5 FISH coupled to HuC IHC on a section of 3 days post lesion (dpl) adult zebrafish telencephalon. Inset is magnified image. cxcr5 is expressed in neurons before and after injury. (C) Quantification graph for cxcr5-expressing HuC-positive cells. (D)cxcr5 chromogenic in situ hybridization (ISH) coupled to NeuroD IHC in unlesioned telencephalon. NeuroD-positive differentiating neurons, which are several cell diameters away from the ventricle express cxcr5 (white asterisks). cxcr5 expression is weaker in NeuroD-positive neurons in comparison to cxcr5-positive cells closer to the ventricle (yellow asterisks). In a transition zone between strong NeuroD-positive (white asterisks) and NeuroD-negative cells (yellow asterisks), cells express NeuroD and cxcr5 weakly (blue asterisks). (E)cxcr5 chromogenic ISH coupled to NeuroD IHC in 3 dpl telencephalon. NeuroD-positive differentiating neurons are more numerous, dispersed inside the parenchyma distantly in comparison to unlesioned telencephalons and express cxcr5 (white asterisks). cxcr5 expression is weaker in NeuroD-positive neurons in comparison to cxcr5-positive cells closer to the ventricle (yellow asterisks). (F) Quantification graph for cxcr5-positive NeuroD-expressing cells. The number of NeuroD/cxcr5 double-positive cells increase upon injury in adult zebrafish telencephalon. Scale bars 25 μm; n = 4 telencephalons for every set of analyses. EXPRESSION / LABELING:
|
|
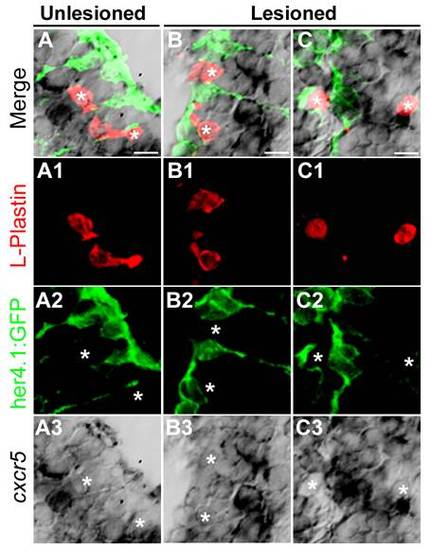
cxcr5 is not expressed in L-Plastin-positive cells in the adult zebrafish telencephalon. (A) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin (red, marking macrophages and microglia) and her4.1:green fluorescent protein (GFP) (green, marking the radial glial cells) in the unlesioned telencephalons. White asterisks indicate the L-Plastin cells. (A1-A3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. (B) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin and her4.1:GFP in the lesioned telencephalons (dorsolateral region). White asterisks indicate the L-Plastin cells. (B1-B3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. (C) cxcr5 in situ hybridization coupled to immunohistochemistry for L-Plastin and her4.1:GFP in the lesioned telencephalons (dorsomedial region). White asterisks indicate the L-Plastin cells. (C1-C3) Individual channels for L-Plastin, her4.1:GFP and cxcr5, respectively. EXPRESSION / LABELING:
|
|
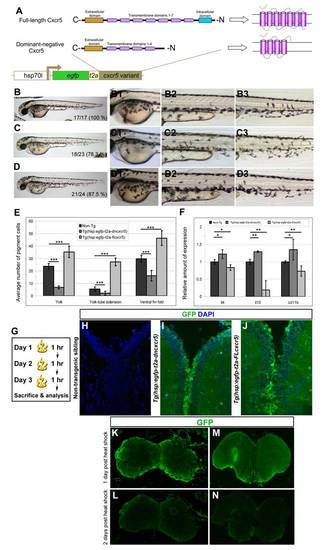
Larval phenotypes upon cxcr5 misexpression; and transgene activity in the adult zebrafish telencephalon. (A) Cxcr5 is a seven-span transmembrane protein with an extracellular receptor domain, seven transmembrane domains and a C-terminus intracellular domain. We generated full-length and dominant-negative versions of Cxcr5. Dominant negative variant lacks the last three transmembrane domains and the intracellular domain. Both variants are inserted into a transgenesis cassette that contains the coding sequence for enhanced green fluorescent protein (EGFP) and self-cleaving T2A peptide. The whole cassette is expressed under heat-inducible hsp70l promoter. (B) Non-transgenic sibling at 3 days post-fertilization (dpf). (B1) High-magnification image of the yolk-sac in B. (B2) High-magnification image of the yolk-tube extension in B. (B3) High-magnification image of the ventral fin fold in B. (C)Tg(hsp:egfp-T2A-dncxcr5) transgenic animals at 3 dpf after two heat shocks in gastrula. (C1) High-magnification image of the yolk-sac in C. (C2) High-magnification image of the yolk-tube extension in C. (C3) High-magnification image of the ventral fin fold in C. (D)Tg(hsp:egfp-T2A-FLcxcr5) transgenic animals at 3 dpf after two heat shocks in gastrula. (D1) High-magnification image of the yolk-sac in D. (D2) High-magnification image of the yolk-tube extension in D. (D3) High-magnification image of the ventral fin fold in D. (E) Quantification graph for the number of pigment cells in different regions of the non-transgenic, Tg(hsp:egfp-T2A-dncxcr5) and Tg(hsp:egfp-T2A-FLcxcr5) larvae. Note that dominant negative variant of cxcr5 significantly reduces while full-length cxcr5 significantly increases the number of pigment cells in comparison to the non-transgenic siblings. (F) Quantitative real-time PCR analyses at 3 dpf after the misexpression of cxcr5 with two heat shocks at gastrula. (G) Heat-shock scheme for adult zebrafish telencephalon expression. (H) GFP and DAPI staining in heat-shocked non-transgenic animals. (I) GFP and DAPI staining in heat-shocked Tg(hsp:egfp-T2A-dncxcr5) animals. (J) GFP and DAPI staining in heat-shocked Tg(hsp:egfp-T2A-FLcxcr5) animals. (K-M) GFP IHC on Tg(hsp:egfp).(K) Olfactory bulb at 1 day after heat-shock. (L) Olfactory bulb at 2 days after heat-shock. (M) Telencephalon at 1 day after heat-shock. (N) Telencephalon at 2 days after heat-shock. GFP protein as a result of heat shock paradigm reduces significantly after 24 hours after heat shock. |
|
cxcr5 translation-blocking antisense morpholino is functional. (A) Uninjected 2 day post-fertilization (dpf) embryos. (B) Control morpholino-injected embryos show no morphological phenotypes at 2 dpf. (C)cxcr5 antisense morpholinoinjected embryos display severe anomalies in axial extension and head development. (D)cxcr5 mRNA rescues the knockdown phenotypes when co-injected with antisense morpholinos. (E) Quantitative real-time PCR analysis of genes regulated by cxcr5, upon control and antisense morpholino injections. All the genes tested are upregulated after knocking down cxcr5. Percentages in A-D represent the ratio of embryos with gross morphological defects to the whole clutch size. |
|
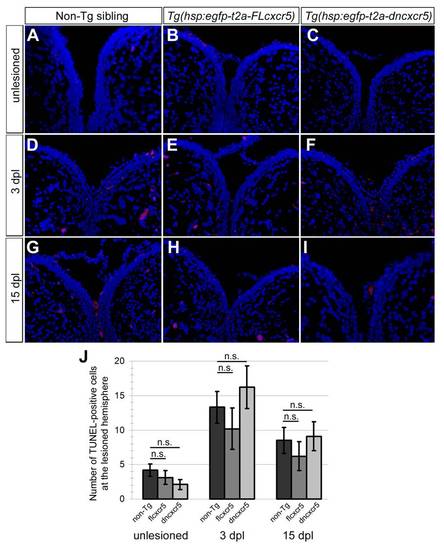
Misexpression of cxcr5 does not lead to cell death. (A-I) TUNEL staining to detect the apoptotic cells in non-transgenic siblings, Tg(hsp:egfpt2a-FLcxcr5) and Tg(hsp:egfp-t2a-dncxcr5) animals pre-lesion, 3 days post-lesion (dpl) and 15 dpl time points. Red nuclei indicate the apoptotic cells. (J) Quantification graph shows the number of apoptotic nuclei in the lesioned hemisphere. Misexpression of cxcr5 does not alter the levels of cell death. Two telencephalons were used for every time point. |