- Title
-
The role of hair cells, cilia and ciliary motility in otolith formation in the zebrafish otic vesicle
- Authors
- Stooke-Vaughan, G.A., Huang, P., Hammond, K.L., Schier, A.F., and Whitfield, T.T.
- Source
- Full text @ Development
|
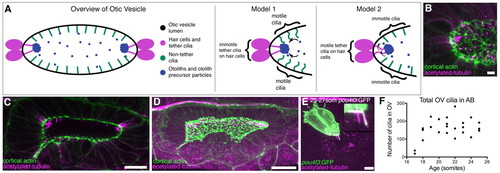
Presence of cilia in the developing otic vesicle of wild-type zebrafish embryos. (A) Cartoon overview of 18-25S OV and illustrations of current models of otolith formation. (B) Confocal projection showing the anterior OV pole in a 21S embryo (lateral view), stained with FITC-phalloidin (green; cortical actin) and anti-acetylated tubulin (magenta). Scale bar: 5 μm. (C) Single confocal section showing clusters of cilia at the anterior and posterior poles in a 21S stage AB embryo (dorsal view). Scale bar: 20 μm. (D) Confocal projection of the whole OV in a 19-21S LWT embryo. Scale bar: 20 μm. (E) Anti-GFP and anti-acetylated tubulin stain of a Tg(pou4f3:mGFP) embryo. GFP marks the hair cells and their kinocilia. Tether cilia are double-labelled and appear white; other cilia are magenta (inset). Scale bar: 5 μm. (F) Scatter plot showing change in total number of cilia in the OV of AB wild-type embryos. |
|
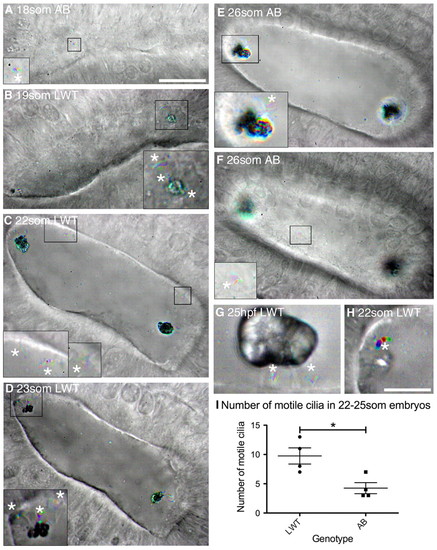
Ciliary motility in the developing wild-type zebrafish otic vesicle. All panels are dorsolateral views of the left ear, with anterior to the right, and are merged time-to-colour composites of six consecutive frames of a high-speed movie. Grey scale shows lack of movement; colour indicates movement. White asterisks mark motile cilia. (A) 18S AB strain wild-type embryo with one motile cilium present on the medial wall (shown in inset). See supplementary material Movie 1. (B) 19S LWT wild-type embryo. Inset shows motile cilia next to the anterior otolith and movement of the anterior otolith. See supplementary material Movie 2. (C) 22S LWT wild-type embryo. Insets: motile cilia present on the medial wall of the OV (two in the left hand inset, one in the right hand inset). See supplementary material Movie 3. (D) 23S LWT wild-type embryo. The posterior otolith is shown on two immotile tether cilia (grey); three motile cilia are nearby (coloured). See supplementary material Movie 4. (E) 26S AB wild-type embryo. Inset: motile cilia next to posterior otolith. See supplementary material Movie 5. (F) Different focal plane of the embryo shown in E. One motile cilium was present on the medial wall of the OV (inset) that rotated first one way and then the other. See supplementary material Movie 6. (G) 25 hpf LWT wild-type embryo. The otolith sits on two to three tether cilia; two short, motile cilia remain directly underneath. See supplementary material Movie 7. (H) Very rarely, one or a few otolith precursor particles were bound to a motile cilium (asterisk; 22S LWT wild-type embryo). See supplementary material Movie 8. (I) Number of motile cilia found per OV in AB and LWT wild-type strains. Bars represent mean ± s.e.m.. Scale bars: 20 μm in A, for A-F; 10 μm in H, for G,H and insets in A-F. |
|
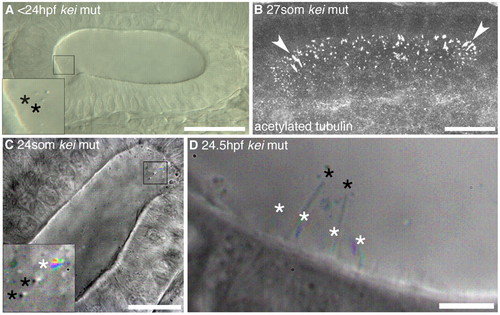
Tether cilia are immotile even in the absence of otoliths. (A) DIC image of a <24 hpf kei mutant showing anterior tether cilia (inset, black asterisks) without any bound otolith, although otolith precursor particles are present. Scale bar: 40 μm. (B) Confocal stack through the OV of a 27S kei mutant embryo. Cilia appear normal (arrowheads indicate tether cilia). Scale bar: 20 μm. (C) Time-to-colour merge of six frames of a high-speed movie of a 24S kei mutant embryo. Inset: two immotile kinocilia (marked by black asterisks) and one motile cilium (marked by white asterisk). Scale bar: 20 μm. See supplementary material Movie 9. (D) Time-to-colour merge of six frames of a high-speed movie of a 24.5 hpf kei mutant embryo; kinocilia (black asterisks) are immotile. White asterisks mark motile cilia. Scale bar: 6 μm. See supplementary material Movie 10. |
|
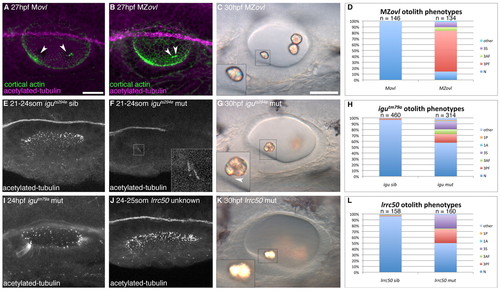
Formation of otoliths in the absence of cilia. (A) Anti-acetylated tubulin and FITC-phalloidin stain showing tether cilia in an Movl embryo (arrowheads; magenta). (B) MZovl mutant ears lack all cilia, but stereociliary bundles are still present (arrowheads; green). (C) Imperfect otoliths form in the correct position in MZovl. Inset: otolith resting directly on a stereociliary bundle. (D) Graph of otolith defects in MZovl embryos. (E) Confocal stack showing cilia in a phenotypically wild-type iguts294e sibling embryo. (F) Anterior kinocilia present in an iguts294e mutant (inset). (G) The iguts294e mutant lacks most cilia but still has some tether cilia (inset, arrowhead). (H) Graph of otolith defects in igutm79a. (I) The igutm79a mutant allele has more otic cilia than the iguts294e allele, but fewer than in wild-type embryos. (J) Representative confocal stack of an ear from an lrrc50 cross. It was not possible to distinguish lrrc50 mutant from sibling embryos at <24 hpf (n=15 ears). (K) DIC image of the ear of an lrrc50 mutant. (L) Graph of lrrc50 mutant otolith phenotypes. N, normal (two otoliths, in correct positions); 1A, one anterior otolith; 1P, one posterior otolith; 3S, three separate otoliths; 3AF, three otoliths, with two anterior otoliths fused; 3PF, three otoliths, with two posterior otoliths fused; other, other otolith defects. Scale bars: 25 μm in A, for A,B,E,F,I,J; 30 μm in C, for C,G,K. |
|
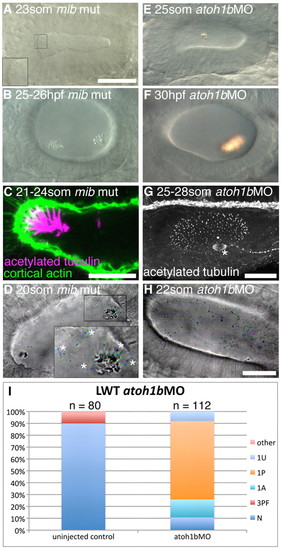
Hair cells are essential for normal otolith formation. (A) Live image of a mib mutant ear at 23S. Inset: ectopic tether cilia. (B) Live image of a 25-26 hpf mib mutant ear. Otoliths do not form correctly. (C) Confocal image showing ectopic tether cilia in a mib mutant ear at 21-24S. (D) Time-to-colour merge of six frames from a high-speed movie of a mib mutant ear. Initially, all ectopic tether cilia nucleate the otolith; motile cilia are present (white asterisks). See supplementary material Movie 12. (E) Live image of atoh1bMO-injected embryo at 25S. Hair cells are absent; an untethered otolith has formed in the middle of the ear. (F) Live image of atoh1bMO-injected embryo at 30 hpf. The single otolith has become tethered to hair cell kinocilia that developed after initial otolith formation. (G) Confocal stack (anti-acetylated tubulin stain) showing presence of non-tether cilia in the ear of an atoh1b MO-injected embryo. The spindle of a dividing cell is visible (asterisk). (H) Time-to-colour merge of six frames of a high-speed movie of an ear from an atoh1b morphant embryo, showing a greater number of untethered otolith precursor particles, and absence of tether cilia and otoliths. Motile cilia are still present (n=3 ears; cf. Fig. 2C). See supplementary material Movie 13. (I) Otolith defects in the ears of atoh1b morphant embryos. N, normal (two otoliths, in correct positions); 1A, one anterior otolith; 1P, one posterior otolith; 1U, one untethered otolith; 3PF, three otoliths, with two posterior otoliths fused; other, other otolith defects. Scale bar: 40 μm in A, for A,B,E,F; 10 μm in C; 25 μm in G; 20 μm; in H, for D,H. |
|
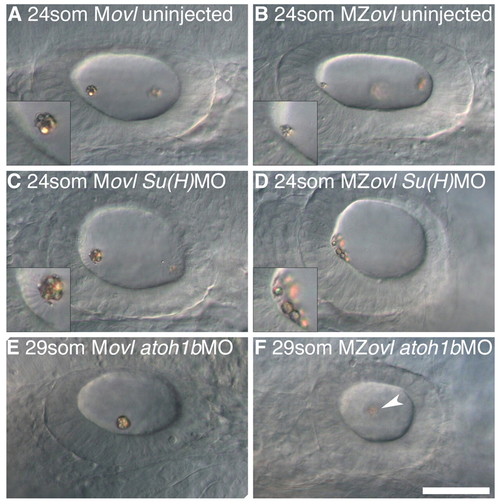
The effect of ectopic hair cells and loss of hair cells on otolith formation in the absence of cilia. All panels show left ear with anterior to the left. (A) 24S uninjected Movl control embryo; inset shows anterior otolith on tether cilia. (B) 24S uninjected MZovl mutant embryo; inset shows small anterior otolith tethered to the hair cells’ apical surface at the anterior OV pole. (C) 24S Movl control embryo injected with Su(H)1+2MO; inset shows otolith bound to several ectopic tether cilia. (D) 24S MZovl mutant embryo injected with Su(H)1+2MO; inset shows otolith precursor particles tethered to the apical surface of the ectopic hair cells. (E) 29S Movl control embryo injected with atoh1bMO; one untethered otolith is present in the ear. (F) 29S MZovl mutant embryo injected with atoh1bMO; one untethered otolith (slightly out of focal plane) is present in the ear (arrowhead). The ovl genotype was confirmed for all samples by genotyping (data not shown). Scale bar: 40 μm for A-F. |
|
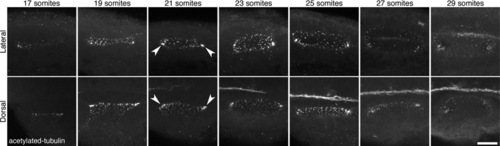
Presence of cilia in the developing otic vesicle of wild-type zebrafish embryos. Stage series showing cilia, marked by an antibody to acetylated tubulin, in the developing otic vesicle (OV) of wild-type (AB) embryos between 17S and 29S. Arrowheads mark denser ciliated patches at the anterior and posterior poles. All panels are maximum confocal projections through the entire OV. Scale bar: 25 μm. |
|
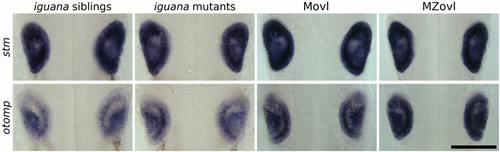
Expression of genes required for otolith development is not affected in cilia mutants. There was no difference in expression of stm and otomp between igu mutants and siblings and MZovl and sibling Movl embryos at 26 hpf. Scale bar: 100 μm. |