- Title
-
Retinoic Acid signaling plays a restrictive role in zebrafish primitive myelopoiesis
- Authors
- Liang, D., Jia, W., Li, J., Li, K., and Zhao, Q.
- Source
- Full text @ PLoS One
|
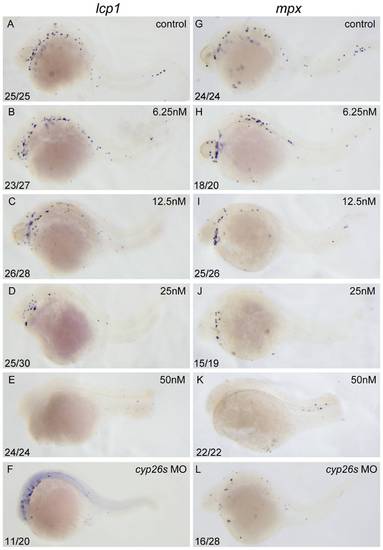
Excessive RA inhibits primitive myelopoiesis in zebrafish embryos in a dose dependent manner. All embryos are positioned anterior left and lateral front. Embryos were treated with vehicle DMSO (A, G), 6.25 nM (B, H), 12.5 nM (C, I), 25 nM (D, J) and 50 nM RA (E, K) respectively from 1–2-cell stage until 26 hpf or microinjected with cyp26a1-MO, cyp26b1-MO and cyp26c1-MO together at 1–2-cell stage (F, L). They were then examined for expressions of myeloid markers lcp1 (A–F) and mpx (G–L) at 26 hpf by whole mount in situ hybridization. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. |
|
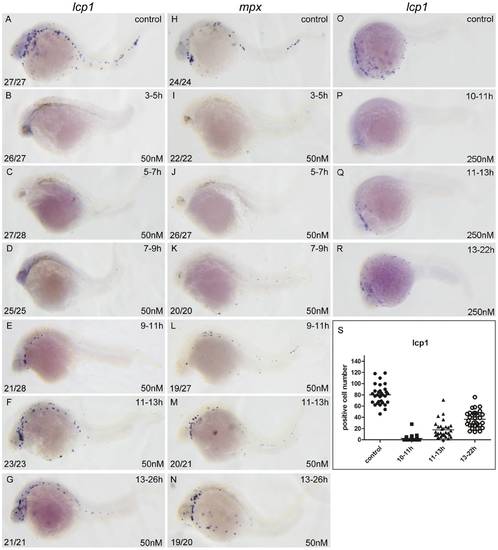
RA restricts the primitive myelopoiesis mainly before 11 hpf. All embryos are positioned anterior left and lateral front. Embryos were treated with vehicle DMSO (A, H, O) or with 50 nM RA (B–G, I–N) from 3 to 5 (B, I), 5 to 7 (C, J), 7 to 9 (D, K), 9 to 11 (E, L), 11 to 13 (F, M) and 13 to 26 hpf (G, N), or with 250 nM RA form 10 to 11 (P), 11 to 13 (Q), 13 to 22 hpf (R), respectively. They were then examined for expressions of myeloid markers lcp1 (A–G, O–R) and mpx (H–N) at 26 hpf (A–N) or 22 hpf (O–R) by whole mount in situ hybridization. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. The typical embryos expressing lcp1+ cells at 22 hpf were shown in O–R. The scatter plot (S) shows the number of lcp1+ cells counted from each of the embryos at 22 hpf with different treatment (control; 10–11 hpf RA treatment; 11–13 hpf RA treatment and 13–22 hpf RA treatment). |
|
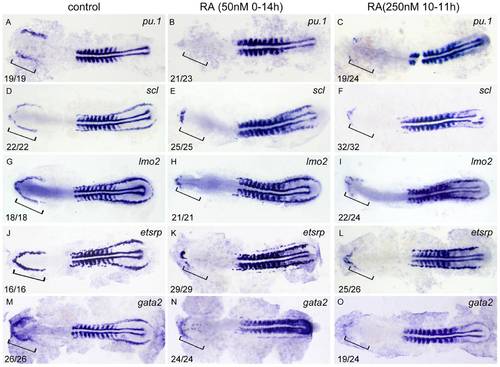
RA restricts the formation of anterior hemangioblasts in zebrafish embryos. All flat-mounted embryos are positioned anterior left and dorsal front. Embryos were treated with vehicle DMSO (A, D, G, J, M), 50 nM RA from 1–2-cell stage to 14 hpf (B, E, H, K, N) or 250 nM RA from 10 to 11 hpf (C, F, I, L, O), respectively. They were then examined for expressions of pu.1 (A–C), scl (D–F), lmo2 (G–I), etsrp (J–L), and gata2 (M–O) in the rostral end of ALPM at 14 hpf by whole mount in situ hybridization. Expression of myoD in somites is used for staging. Bracket indicates the location of RBI. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. |
|
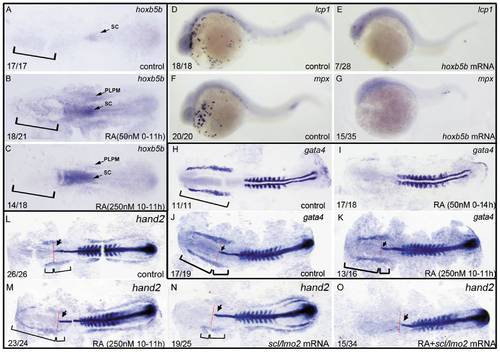
ALPM is lost in the embryos treated with 50 nM RA from 1–2-cell stage to 11 hpf but not eliminated in the ones treated with 250 nM RA during 10–11 hpf. All embryos including flat-mounted embryos (A–C, H–O) and whole-mounted embryos (D–G) are positioned anterior left and dorsal front (A–C, H–O) or lateral front (D–G). Embryos treated with vehicle DMSO (A), 50 nM RA from 1–2-cell stage to 11 hpf (B), and 250 nM RA during 10–11 hpf (C) were examined for hoxb5b expression at 11 hpf (A–C). Embryos treated with 50 nM RA treatment from 1–2-cell stage to 11 hpf displayed ectopically expression of hoxb5b in ALPM (B) but the ones treated with 250 nM RA during 10–11 hpf did not exhibit this ectopical expression (C). Compared with control embryos (D, F), overexpressions of hoxb5b by microinjecting embryos at 1–2-cell stage with hoxb5b mRNA significantly suppressed expressions of lcp1 (E) and mpx (G) at 24 hpf. Embryos treated with vehicle DMSO (H, J), 50 nM RA from 1–2-cell stage to 14 hpf (I) or 250 nM RA during 10–11 hpf (K) were examined for expressions of ALPM marker gata4 at 14 hpf. The location of ALPM at 11 hpf (A–C) or at 14 hpf (H, J, K) is indicated by bracket. Embryos treated with vehicle DMSO (L), 250 nM RA during 10–11 hpf (M, O), or microinjected with scl/lmo2 mRNA (N, O) were examined for expressions of cardiac marker hand2 at 14 hpf. Expression of myoD in somites (H–O) was used for staging and ntl expression was used for labeling embryonic axial mesoderm (J–O). The length between the anterior end of gata4 expression domain (J, K) or hand 2 expression domain (L–N) and the anterior end of ntl expression domain and the length between the posterior end of gata4 expression domain (J, K) or hand 2 expression domain (L–N) and the anterior end of ntl expression domain are marked by two different brackets. Red line denotes the anterior level of ntl expression domain (J–O). Arrow indicates the most anterior end of notochord marked by expression of ntl (J–O). sc: spinal cord; PLPM: posterior lateral plate mesoderm. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. |
|
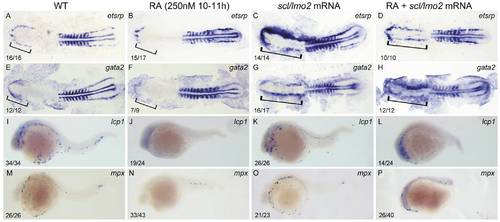
Overexpressions of scl and lmo2 into RA-treated zebrafish embryos partially rescue the defective primitive myelopoiesis. Both flat-mounted embryos (A–H) and whole-mounted embryos (I–P) are positioned anterior left and dorsal front (A–H) or lateral front (I–P). Embryos were treated with vehicle DMSO (A, E, I, M), 250 nM RA during 10 to 11 hpf (B, F, J, N), or microinjected with scl and lmo2 mRNA at 1–2-cell stage (C, G, K, O), or microinjected with scl and lmo2 mRNA at 1–2-cell stage and then treated with 250 nM RA during 10 to 11 hpf (D, H, L, P), respectively. They were then examined for expressions of hemangioblast markers etsrp (A–D) and gata2 (E–H) at 14 hpf, and myeloid markers lcp1 (I–L) and mpx (M–P) at 24 hpf by whole mount in situ hybridization. Expression of myoD in somites was used for staging (A–H). Bracket indicates the location of RBI (A, B, E, F), and ALPM (C, D, G, H). The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. |
|
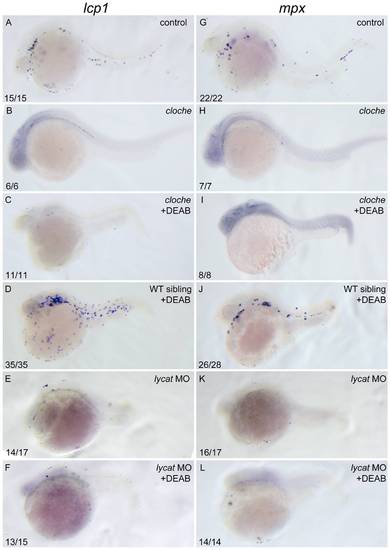
DEAB cannot rescue the defective primitive myelopoiesis in cloche or lycat knockdown embryos. All embryos are positioned anterior left and lateral front. Wild type siblings (A, G), cloche (B, H) and the embryos microinjected with lycat-MO at 1–2-cell stage (E, K) were treated with vehicle DMSO whereas cloche (C, I), cloche siblings (D, J) and lycat-MO knockdown (F, L) embryos were treated with 10 μM DEAB from 1–2-cell stage until 26 hpf. They were then examined for expressions of myeloid markers lcp1 (A–F) and mpx (G–L) at 26 hpf by whole mount in situ hybridization. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. |
|
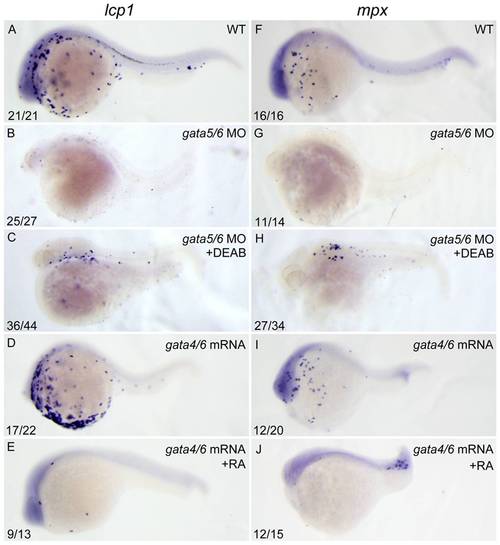
RA inhibits primitive myelopoiesis by acting downstream of gata4/5/6. All embryos are positioned anterior left and lateral front. Embryos were microinjected with gata5-MO and gata6-MO together (B, C, G, H) at 1–2-cell stage and then treated with 10 μM DEAB (C, H) or vehicle DMSO (B, G) immediately, or microinjected with gata4 mRNA and gata6 mRNA together (D, E, I, J) and then treated with 250 nM RA (E, J) or vehicle DMSO (D, I) from 10 to 11 hpf, respectively. They were then examined for expressions of myeloid markers lcp1 (A–E) and mpx (F–J) at 24 hpf by whole mount in situ hybridization. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. |
|
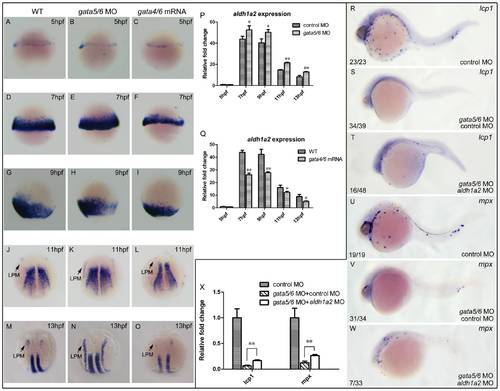
aldh1a2 is one of downstream target genes of gata4/5/6 to affect zebrafish primitive myelopoiesis. Embryos are positioned animal pole top and ventral front (A–I), anterior top and dorsal front (J–O), and anterior left and lateral front (R–W). Embryos were microinjected with control MO (A, D, G, J, M, R, U), gata5-MO plus gata6-MO (B, E, H, K, N), gata4 mRNA plus gata6 mRNA (C, F, I, L, O), gata5-MO and gata6-MO plus control MO (S, V), gata5-MO and gata6-MO plus aldh1a2 MO (T, W) at 1–2-cell stage, respectively. They were then examined for expressions of aldh1a2 at 5 hpf (A–C), 7 hpf (D–F), 9 hpf (G–I), 11 hpf (J–L) and 13 hpf (M–O), lcp1 (R–T) and mpx at 24 hpf (U–W) by whole mount in situ hybridization, respectively. qRT-PCR was performed to confirm the relative expression level changes of aldh1a2 in gata4/5/6 depleted embryos (P) or in gata4/6 overexpressed embryos (Q) at 5, 7, 9, 11, 13 hpf, and those of lcp1 and mpx at 24 hpf (X). The number shown in the lower left-hand corner of each panel (R–W) is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. *: P<0.05; **: P<0.01. |
|
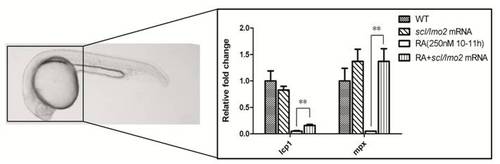
qRT-PCR analysis shows overexpression of scl and lmo2 into zebrafish embryos partially rescues the defective primitive myelopoiesis in the embryos treated with 250 nM RA during 10–11 hpf. Embryos were treated with vehicle DMSO (WT), microinjected with scl and lmo2 mRNA at 1–2 cell stage (scl/lmo2 mRNA), treated with 250 nM RA during 10 to 11 hpf (250 nM RA 10–11 hpf), or microinjected with scl and lmo2 mRNA at 1–2-cell stage and then treated with 250 nM RA during 10 to 11hpf (RA+scl/lmo2 mRNA), respectively. To exclude the myeloid cells derived from ICM, embryos that were removed tails and trunks at 24 hpf were used to detect relative expression levels of myeloid markers lcp1 and mpx by qRT-PCR. Overexpression of scl and lmo2 did not change expression levels of lcp1 and mpx in wild type embryos but significantly rescued the inhibited expressions of lcp1 and mpx in the embryos treated with 250 nM during 10–11 hpf. **: P<0.01. |
|
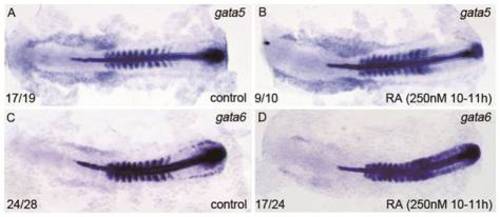
Effect of excessive RA treatment during 10–11 hpf on expressions of gata5 and gata6 in the RA-treated embryos at 14 hpf. All flat-mounted embryos are positioned anterior left and dorsal front. Embryos treated with vehicle DMSO (A, C) and 250 nM RA from 10–11 hpf were examined for expressions of gata5 (A, B) and gata6 (C, D) at 14 hpf, respectively. Expression of myoD in somites was used for staging and ntl expression was used for labeling embryonic axial mesoderm. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. The embryos treated with 250 nM RA from 10–11 hpf exhibited similar expression of gata5 but somehow increased expression of gata6 in the region between the anterior ends of their expression domains and the anterior end of ntl expression domain; however, they displayed significantly increased gata5 expression but greatly reduced gata6 expression in the region between the posterior ends of their expression domains and the anterior end of ntl expression domain, respectively. |
|
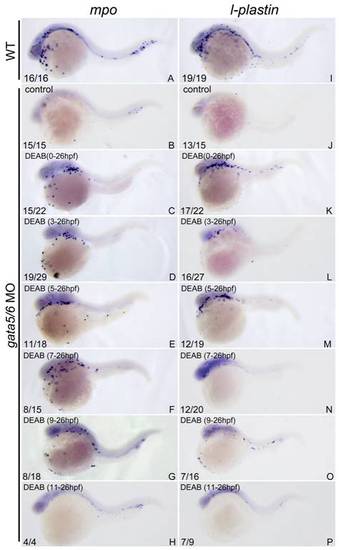
The ablated primitive myelopoiesis in gata4/5/6-depleted embryos can be rescued by treating with 10 μM DEAB starting before 11 hpf. All embryos were positioned anterior left and lateral front. Embryos were microinjected with gata4-MO and gata6-MO at 1–2-cell stage (B–H, J–P) and then treated with vehicle DMSO (B, J) or 10 μM DEAB continuously starting from 1–2-cell stage (C, K), 3 hpf (D, L), 5 hpf (E, M), 7 hpf (F, N), 9 hpf (G, O), and 11 hpf (H, P), respectively. The embryos were then grown together with wild type control embryos (A, I) to 24 hpf for examining expressions of myeloid markers lcp1 (A–H) and mpx (I, P) respectively. The number shown in the lower left-hand corner of each panel is the number of embryos exhibiting the typical phenotype shown in the panel to the number of embryos totally observed. Treatment with DEAB before 11 hpf can well rescue the abolished primitive myelopoiesis in gata4/5/6-depleted embryos (C–G; K–O) whereas the treatment after 11 hpf hardly rescued the primitive myelopoiesis (H, P). |