- Title
-
HB-EGF Is Necessary and Sufficient for Müller Glia Dedifferentiation and Retina Regeneration
- Authors
- Wan, J., Ramachandran, R., and Goldman, D.
- Source
- Full text @ Dev. Cell
|
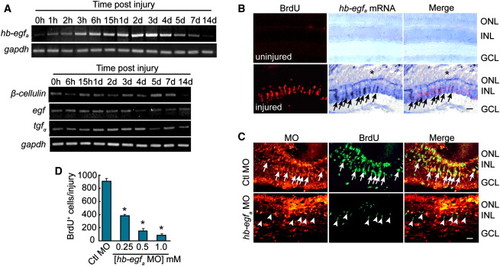
Injury-Dependent hb-egfa Induction Is Necessary for MG Dedifferentiation and Proliferation (A) RT-PCR showing the temporal expression pattern of genes encoding EGFR ligands in the uninjured and injured retina. (B) In situ hybridization and BrdU immunofluorescence show that hb-egfa is induced in proliferating progenitors in the injured retina (arrows) (* marks the injury site). (C) MO-mediated HB-EGFa knockdown reduces the number of proliferating MG-derived progenitors in the injured retina at 4 dpi. Arrows identify proliferating cells in the control MO-treated retina, whereas arrowheads identify proliferating cells in the HB-EGFa knockdown retina (note that these latter cells lack lissamine-labeled MO). (D) Quantification of (C) at different hb-egfa MO concentrations. *p < 0.01. Error bars represent SD. Scale bars, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. |
|
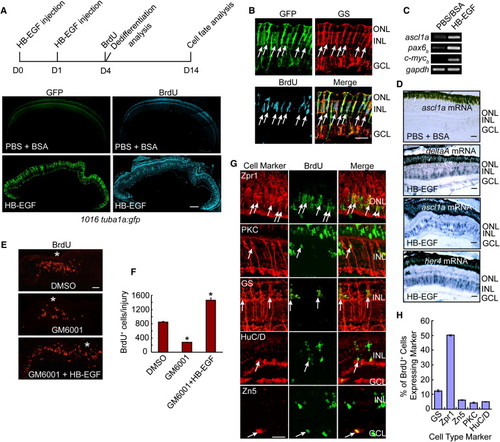
HB-EGF Stimulates MG Dedifferentiation into Multipotent Retinal Progenitors in the Uninjured Retina (A) Diagram of experimental protocol and low-magnification photomicrographs showing that HB-EGF injection into the eye of 1016 tuba1a:gfp fish stimulates GFP expression and BrdU incorporation throughout the INL of the uninjured retina. Scale bar, 150 μm. (B) High-magnification view of (A) showing that GFP+/BrdU+ cells express the MG marker GS (arrows). Scale bar, 50 μm. (C and D) RT-PCR (C) and in situ hybridization assays (D) showing that HB-EGF stimulates the expression of genes characteristic of dedifferentiated MG. In (D), the PBS plus BSA-treated retinas did not show an in situ hybridization signal above background for any of the probes tested—data only shown for the ascl1a probe. Scale bars, 50 μm. (E) The matrix metalloprotease inhibitor, GM6001, suppresses injury-dependent proliferation of MG-derived progenitors, and this regulation is rescued by soluble HB-EGF. Asterisk (*) identifies the injury site. (F) Quantification of the effects observed in (E). *p < 0.01. (G) BrdU and retinal cell type-specific immunofluorescence shows that HB-EGF-generated progenitors in the uninjured retina at 14 days postinjection are multipotent and generate all major retinal cell types. Scale bar, 50 μm. (H) Quantification of regenerated cell types observed in (G). Error bars represent SD. Scale bars, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. See also Figures S1 and S2. EXPRESSION / LABELING:
|
|
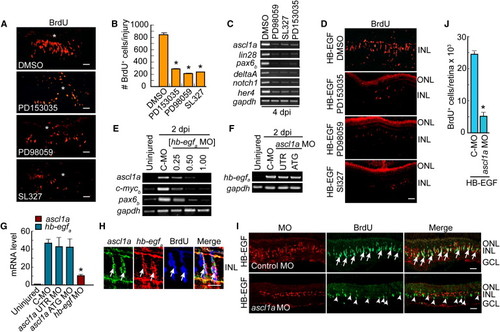
HB-EGF and Retinal Injury Stimulate MG Dedifferentiation via an EGFR/MAPK/Ascl1a-Signaling Cascade (A) EGFR inhibition (PD153035) or MAPK inhibition (PD98059, SL327) reduces the number of proliferating progenitors generated following retinal injury. Asterisks identify the injury sites. (B) Quantification of (A). **p < 0.01. (C) RT-PCR shows that EGFR or MAPK inhibition blocks induction of genes associated with MG dedifferentiation. (D) EGFR or MAPK inhibition reduces the number of proliferating progenitors generated following HB-EGF treatment of the uninjured retina. (E) HB-EGFa knockdown suppresses induction of regeneration-associated genes in the injured retina. [hb-egf MO] is in mM. C-MO is 0.5 mM. (F) Ascl1a knockdown has little effect on hb-egfa induction in the injured retina (MOs are 0.25 mM). (G) Real-time PCR quantification of (E) and (F) normalized to uninjured value. The hb-egf MO is at 0.5 mM, and ascl1a MOs are at 0.25 mM. (H) ascl1a and hb-egfa in situ hybridization assays along with BrdU immunofluorescence show colocalization (arrows) in the injured retina. (I) MO-mediated knockdown of Ascl1a in the presence of HB-EGF suppresses HB-EGF-dependent generation of BrdU+ progenitors. BrdU+ progenitors that do form in the Ascl1a knockdown retina generally lack lissamine-labeled MO (arrowheads). Control MO does not block the generation of BrdU+ progenitors (arrows). (J) Quantification of BrdU+ cells in (I). *p < 0.01. Error bars represent SD. All scale bars, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. See also Figure S3. EXPRESSION / LABELING:
|
|
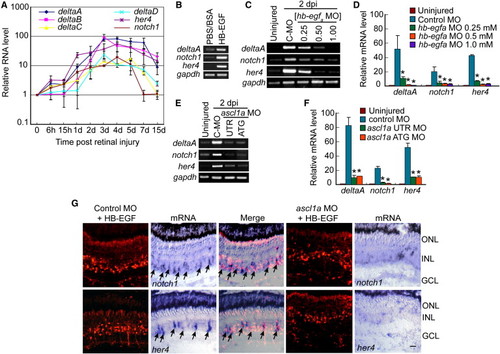
HB-EGF and Ascl1a Regulate Notch-Signaling Component Genes in the Injured Retina (A) Real-time PCR shows injury-dependent induction of Notch-signaling components. (B) RT-PCR shows that HB-EGF activates Notch-signaling component genes in the uninjured retina. (C) RT-PCR shows that MO-mediated HB-EGFa knockdown suppresses injury-dependent induction of Notch-signaling component genes. (D) Real-time PCR quantification of (C). *p < 0.01. (E) RT-PCR shows that Ascl1a knockdown suppresses injury-dependent induction of Notch-signaling component genes. (F) Real-time PCR quantification of (E). *p < 0.01. (G) Ascl1a knockdown suppresses HB-EGF-dependent induction of notch1 and her4 mRNAs. Arrows identify notch1 and her4 mRNA expression in control MO-treated cells. Scale bar, 50 μm. Error bars represent SD. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. See also Figures S4 and S5. EXPRESSION / LABELING:
|
|
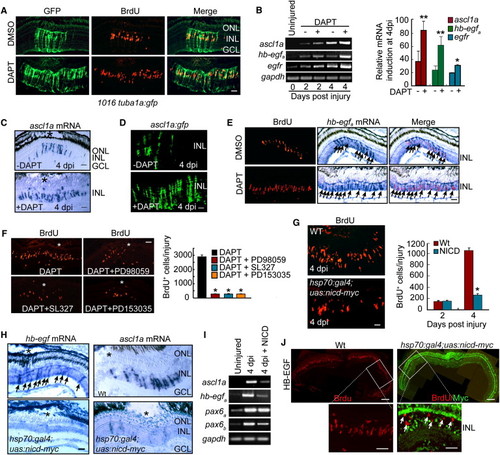
Notch Inhibition Stimulates MG Proliferation and Expands the Zone of Dedifferentiated MG in the Injured Retina via Induction of hb-egfa Gene Expression (A) DAPT treatment of 1016 tuba1a:gfp transgenic fish results in an expansion of the zone of dedifferentiated MG (GFP+/BrdU+) in the injured retina. Scale bar, 50 μm. (B) RT-PCR (gel) and real-time PCR (4 dpi, graph) showing that DAPT induces ascl1a, hb-egfa, and egfr mRNA expression in the injured retina. **p < 0.01 for ascl1a and hb-egfa; *p < 0.05 for egfr. (C) In situ hybridization showing that DAPT expands ascl1a mRNA expression in the injured retina (* marks the injury site). Scale bar, 50 μm. (D) ascl1a:gfp transgenic fish exhibit injury- and Notch-dependent regulation of transgene expression. Scale bar, 50 μm. (E) DAPT stimulates hb-egfa mRNA expression in proliferating MG-derived progenitors (arrows) of the injured retina. Scale bar, 50 μm. (F) MAPK inhibition (PD98059 and SL327) and EGFR inhibition (PD153035) suppress DAPT-dependent expansion of MG proliferation in the injured retina (asterisks in photomicrographs identify injury sites). Graph quantifies data represented in photomicrographs. *p < 0.01. (G) BrdU immunofluorescence on retinal sections reveals BrdU incorporation in the injured retinas of WT and hsp70:gal4;uas:nicd-myc transgenic fish overexpressing NICD. Scale bar, 50 μm. *p < 0.01. (H) In situ hybridization assays for hb-egfa (arrows) and ascl1a mRNA expression in WT and hsp70:gal4;uas:nicd-myc fish overexpressing NICD (* marks the injury site). Scale bar, 50 μm. (I) RT-PCR shows that ascl1a, hb-egfa, and pax6b mRNA expression is inhibited by NICD overexpression. (J) NICD-myc overexpression suppresses HB-EGF-induced cell proliferation in the uninjured retina. BrdU+ cells in HB-EGF-treated retinas overexpressing NICD-myc are generally NICD negative (arrows). Scale bars are 150 μm in low-magnification images and 50 μm in higher-magnification images. Error bars represent SD. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. See also Figure S6. EXPRESSION / LABELING:
|
|
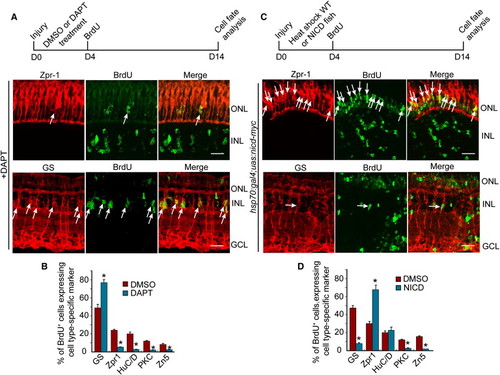
Notch Signaling Regulates the Differentiation of MG-Derived Progenitors (A and C) Diagram of the experimental protocol and representative photomicrographs showing BrdU and retinal cell type-specific immunofluorescence. Arrows identify BrdU and retinal cell type double-labeled cells. Scale bars, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. (B and D) Quantification of double-labeled cell types. *p < 0.01. Error bars represent SD. |
|
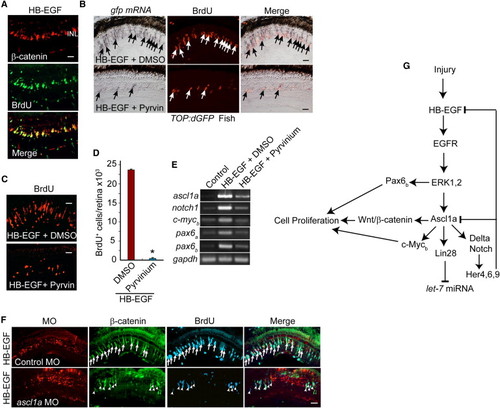
HB-EGF Activates a Wnt/β-Catenin-Signaling Cascade (A) β-Catenin immunofluorescence shows that HB-EGF stimulates β-catenin stabilization in proliferating progenitors of the uninjured retina. (B) In situ hybridization and BrdU immunofluorescence show that HB-EGF-dependent induction of transgene expression in TOP:dGFP Wnt-signaling reporter fish is inhibited by pyrvinium (Pyrvin) (arrows identify double-labeled gfp+/BrdU+ cells). (C and D) Pyrvinium (Pyrvin)-mediated inhibition of Wnt/β-catenin signaling suppresses HB-EGF-dependent generation of proliferating progenitors in the uninjured retina. Error bars represent SD. *p < 0.01. (E) RT-PCR shows that pyrvinium suppresses HB-EGF-dependent induction of regeneration-associated genes in the uninjured retina. (F) Ascl1a knockdown suppresses HB-EGF-dependent β-catenin stabilization and BrdU incorporation in the uninjured retina. Arrows identify β-catenin+ and BrdU+ cells in the control MO-treated retina, whereas arrowheads identify β-catenin+ and BrdU+ cells in the ascl1a MO-treated retina. (G) Summary of signaling cascades and genes regulated by HB-EGF. Scale bars, 50 μm. |
|
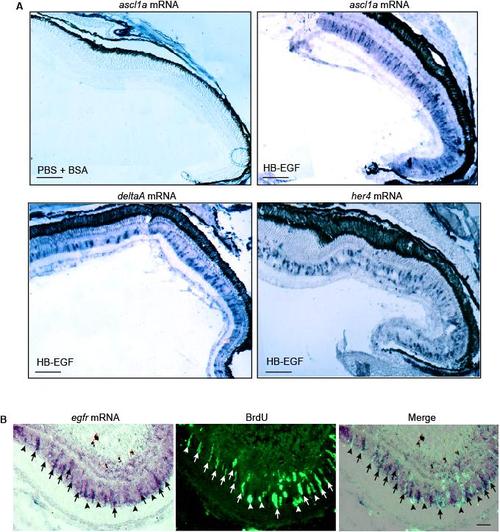
HB-EGF stimulates ascl1a, deltaA and her4 gene expression throughout the uninjured retina. Related to Figure 2. (A) Low magnification view of in situ hybridization assays showing that intravitreal injection of HB-EGF stimulates ascl1a, deltaA and her4 gene expression throughout the retina. In situ hybridization assays for control eyes that only received PBS + BSA showed no expression above background (data shown for ascl1a mRNA, which is representative of the other mRNAs). Scale bar, 150 μm. (B) In situ hybridization and BrdU immunofluorescence shows EGFR mRNA is localized to proliferating MG at the injury site. Arrows identify proliferating MG with strong EGFR expression while arrowheads point to proliferating MG with weak EGFR expression. Scale bar, 50 μm. |
|
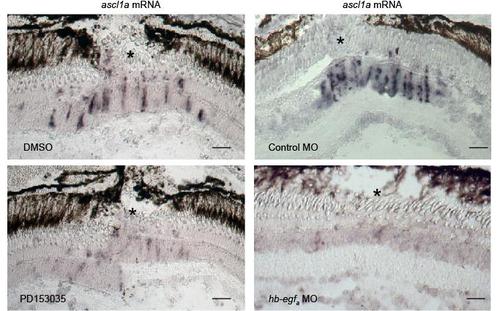
Injury-dependent induction of ascl1a mRNA expression requires HB-EGFa expression and EGFR activation. Related to Figure 3. Adult retinas were injured (* marks the injury site) and the effects of EGFR inhibition with PD153035 or MO-mediated HB-EGFa knockdown on ascl1a mRNA expression was examined using in situ hybridization assays. Scale bar, 50 μm. |
|
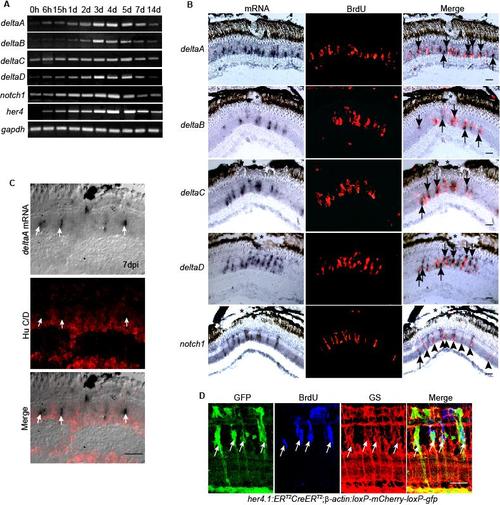
Notch signaling components are induced in proliferating MG-derived progenitors residing in the injured retina. Related to Figure 4. (A) RT-PCR showing the temporal pattern of Notch signaling component gene expression. (B) In situ hybridization combined with BrdU immunofluorescence shows that at 4 dpi genes encoding the Notch ligands, deltaA-D are restricted to the site of injury (*) and are predominantly localized adjacent to BrdU+ progenitors, while Notch receptor, notch1 is predominantly expressed in BrdU+ progenitors at the injury site. (C) In situ hybridization and HuC/D immunofluorescence show deltaA expressing cells are HuC/D-. (D) her4.1:ERT2CreERT2;β-actin:loxP-mCherry-loxP-gfp double transgenic fish show that the Notch responsive promoter, her4, is activated in proliferating MG-derived progenitors identified by BrdU incorporation and glutamine synthetase (GS) expression. Scale bar, 50 μm. |
|
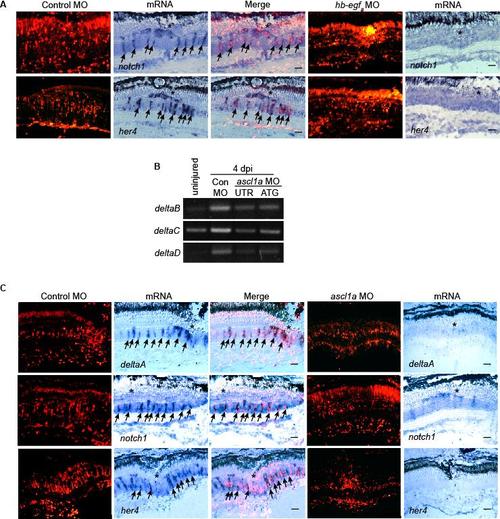
HB-EGFa and Ascl1a are necessary for injury-dependent induction of Notch signaling component genes. Related to Figure 4. (A) In situ hybridization shows MO-mediated HB-EGFa knockdown suppresses injury-dependent induction of notch1 and her4 mRNAs. (B) RT-PCR shows MO-mediated Ascl1a knockdown suppresses injury-dependent induction of deltaB, C and D. (C) In situ hybridization shows Ascl1a knockdown suppresses injury-dependent induction of deltaA, notch1 and her4 mRNAs. Scale bar, 50 μm. |
|
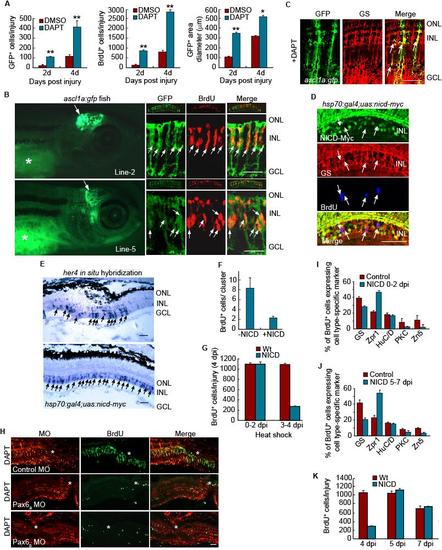
Notch and Pax6-dependent regulation of MG dedifferentiation and retina regeneration. Related to Figure 5. (A) Quantification of GFP+ and BrdU+ cells, along with the area they occupy shown in Figure 5A. **P<0.01, *P<0.05. (B) Four different lines of ascl1a:gfp transgenic fish were generated with each exhibiting transgene expression in the developing midbrain. (2 examples shown in left hand panels). * identifies autofluorescence in the yolk. Right-hand panels show sections through the adult injured retina where transgene expression is confined to BrdU+ progenitors. The small top inset for each panel shows a low magnification view of the retina. (C) In the adult injured retina of ascl1a:gfp fish treated with DAPT, transgene expression is confined to glutamine synthetase (GS)-expressing MG-derived progenitors. Scale bar, 50 μm. (D) NICD-myc, GS and BrdU immunofluoresence at 4 dpi shows NICD over-expression in the injured retina inhibits progenitor proliferation and the few remaining BrdU+ cells do not express NICD. (E) In situ hybridization assays show NICD over-expression stimulates her4 expression throughout the inner nuclear layer. (F) NICD overexpression reduces the number of BrdU+ cells in a neurogenic cluster. (G) NICD overexpression from 0-2 dpi has little effect on progenitor proliferation while NICD overexpression from 3-4 dpi suppresses progenitor proliferation. (H) Pax6a or Pax6b knockdown suppresses DAPT-dependent proliferation of MG-derived progenitors assayed at 4 dpi. (I) Retinal cell types regenerated at 14 dpi following NICD overexpression from 0-2 dpi. (J) Retinal cell types regenerated at 14 dpi following NICD overexpression from 5-7 dpi. (K) Proliferating progenitors remaining after NICD overexpression from 0-4 dpi can expand and contribute to retina regeneration at later times. Error bars are standard deviation. Scale bar, 50 μm. |
Reprinted from Developmental Cell, 22(2), Wan, J., Ramachandran, R., and Goldman, D., HB-EGF Is Necessary and Sufficient for Müller Glia Dedifferentiation and Retina Regeneration, 334-347, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell