- Title
-
Midbrain-hindbrain boundary patterning and morphogenesis are regulated by diverse grainy head-like 2-dependent pathways
- Authors
- Dworkin, S., Darido, C., Georgy, S.R., Wilanowski, T., Srivastava, S., Ellett, F., Pase, L., Han, Y., Meng, A., Heath, J.K., Lieschke, G.J., and Jane, S.M.
- Source
- Full text @ Development
|
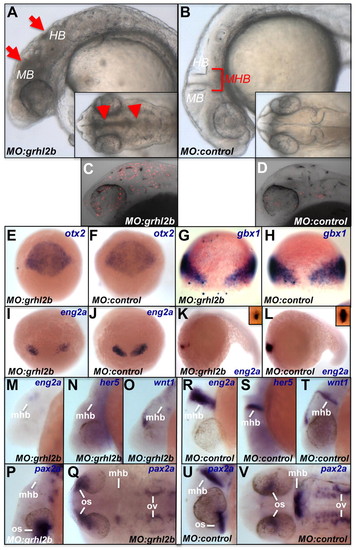
Loss of function of grhl2b leads to severe defects in the MHB region. (A,B) Embryos injected with control or grhl2b ATG-blocking MO. Lateral views with dorsal views in the insets. MB, midbrain; HB, hindbrain; apoptosis and folding defects are indicated by arrows and arrowheads. (C,D) Apoptosis is increased in grhl2b morphants (MO:grhl2b) compared with controls. (E-H) Positioning of either the midbrain (otx2) or hindbrain (gbx1) territories within the neural plate in grhl2b morphants compared with controls. (I-L) Expression of eng2a in grhl2b morphants and controls from the onset of MHB formation. (M-V) Expression of MHB patterning genes (eng2a, her5, wnt1 and pax2a) at 26 hpf in grhl2b morphants and controls. mhb, midbrain-hindbrain boundary; os, optic stalk; ov, otic vesicles. |
|
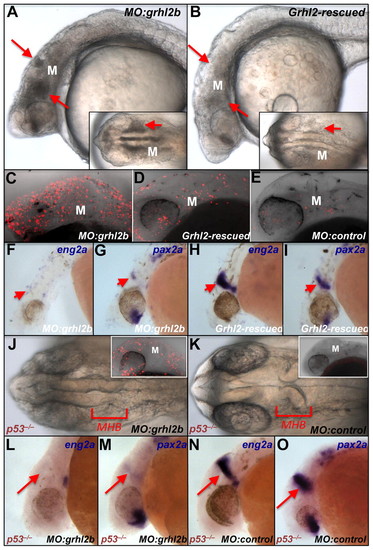
Rescue of neural apoptosis and MHB marker loss in grhl2b morphants by mouse Grhl2 mRNA. (A-E) Cell death rescue (arrows) demonstrated by bright-field microscopy (A,B, insets) and TUNEL staining (C-E) around the MHB region (M) of embryos sequentially injected with MO:grhl2b and either control lacZ mRNA (A,C) or mouse Grhl2 mRNA (B,D). TUNEL staining reveals few apoptotic cells in an embryo injected with MO:control (E). (F-I) Rescue (arrows) of MHB marker expression (eng2a, pax2a) in embryos sequentially injected with MO:grhl2b and either lacZ mRNA (F,G) or mouse Grhl2 mRNA (H,I). (J-O) Persistent defects in MHB folding (J,K) and marker expression (eng2a, pax2a) (L-O, arrows) in p53–/– fish injected with MO:grhl2b compared with MO:control. TUNEL assay confirms substantially reduced apoptosis in p53–/– fish injected with MO:grhl2b (J, inset; compare with C) |
|
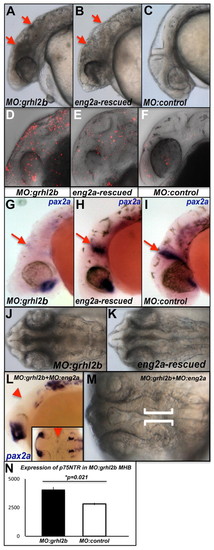
Rescue of neural apoptosis and MHB marker loss in grhl2b morphants by eng2a mRNA. (A-F) Rescue of cell death (arrows) shown by bright-field microscopy (A-C) and TUNEL assay (D-F) in lateral views of 26 hpf embryos sequentially injected with MO:grhl2b and either lacZ mRNA (A,D) or eng2a mRNA (B,E). MO:control embryos injected with lacZ mRNA are shown (C,F). (G-I) Rescue of expression (arrows) of the MHB maintenance marker pax2a in 26 hpf embryos sequentially injected with MO:grhl2b and either lacZ (G) or eng2a (H). MO:control embryo injected with lacZ mRNA is shown (I). (J,K) MHB folding in grhlb2 morphants (J) is not rescued by injection of eng2a mRNA (K). Injection of lacZ (J) as control. (L,M) Cooperativity between grhl2b and eng2a ATG-blocking MOs in inducing loss of pax2a expression and apoptosis (L, arrowhead; dorsal view, inset), but not MHB folding (brackets, M). Lateral views. (N) Q-RT-PCR analysis of MHBs extracted from n=3 MO:grhl2b 24 hpf morphants and MO:control embryos showing expression of p75NTR. |
|
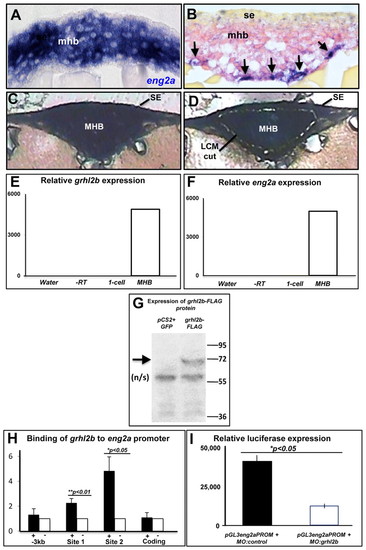
eng2a is a direct target gene of grhl2b in the MHB. (A,B) High-powered coronal cross-sections, showing eng2a (A) and grhl2b (arrows) and pax2a (B) expression in the MHB in wild-type embryos at 12 hpf. mhb, midbrain-hindbrain boundary; se, surface ectoderm. (C,D) Coronal sections of MHB shown before laser capture microdissection (C) and highlighting the excised region (D). (E,F) Q-RT-PCR of grhl2b (E) and eng2a (F) from laser capture microdissection MHB tissue, relative to cDNA from pre-zygotic transcription-stage (one-cell) embryos, water and RT-negative controls. (G) Immunoblot of FLAG-tagged grhl2b (arrow) in cell extracts from HEK-293 cells transfected with pCS2+grhl2b-FLAG or the negative control (pCS2+GFP). n/s, non-specific band. (H) ChIP from embryos expressing FLAG-tagged grhl2b (+) or non-tagged grhl2b (–) using anti-FLAG antibody and primers for the two predicted grhl2b-binding sites in the eng2a promoter. Data are shown as the mean fold enrichment±s.e.m. (Q-RT-PCR) from two independent experiments performed with at least five embryos in each group. Negative control regions of the promoter or exon 1 (′–3 kb′ and ′coding′. respectively) show no significant enrichment. (I) Reporter gene assay using a 3 kb region of the eng2a promoter linked to the luciferase gene (pGL3-eng2a-PROM) in 24 hpf embryos co-injected with either MO:control or MO:grhl2b. Data are shown as mean light units±s.e.m. from two independent experiments performed with a minimum of 40 embryos in each group. EXPRESSION / LABELING:
|
|
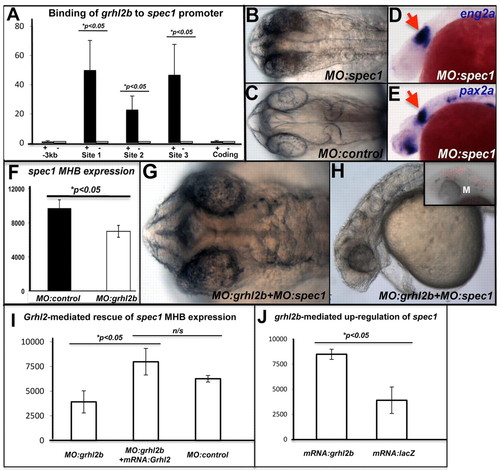
grhl2b cooperates with a novel direct transcriptional target, spec1, to regulate MHB morphogenesis. (A) ChIP from embryos expressing FLAG-tagged grhl2b (+) or non-tagged grhl2b (–) using anti-FLAG antibody and primers to three predicted grhl2b-binding sites in the spec1 promoter. Data are shown as the mean fold-enrichment±s.e.m. (Q-RT-PCR) from three independent experiments with at least five embryos per group. (B,C) Loss of MHB folding in spec1 morphants at 26 hpf. (D,E) Expression of MHB markers (eng2a, pax2a, arrows) persists in spec1 morphants at 26 hpf. (F) Q-RT-PCR of spec1 expression in the MHB of grhl2b morphants. The MHB was dissected from five embryos per group, and the data are presented as the mean±s.d. (G,H) Cooperativity between grhl2b and spec1 ATG-blocking MOs in inducing aberrant MHB folding (G, dorsal view), and mid/hindbrain ventricularisation (H, lateral view). (I,J) spec1 mRNA expression at the MHB is restored following rescue of grhl2b morphants by injection of Grhl2 mRNA (I), and is significantly upregulated in wild-type embryos overexpressing grhl2b mRNA (J). |
|
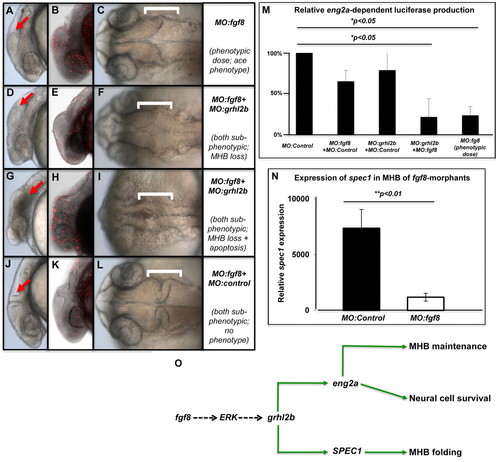
grhl2b is a predicted downstream target of fgf8 signalling. (A-L) Downregulation of fgf8 causes loss of cerebellum and enhanced apoptosis (ace phenotype; A-C), a defect that is recapitulated when fgf8 and grhl2b morpholinos are co-injected at individual sub-phenotypic doses (D-F). More severely affected fgf8-grhl2b double knockdown embryos display enhanced apoptosis and loss of MHB folding in a dose-dependent manner (G-I). No phenotypes or increased apoptosis are seen when fgf8 and control morpholinos are co-injected at sub-phenotypic doses (J-L). Arrows and brackets in all panels show position of the MHB; B,E,H,K show TUNEL staining of the relevant phenotype. (M) Luciferase activity from pGL3-eng2a-PROM is significantly decreased following co-injection of sub-phenotypic doses of MO:fgf8 and MO:grhl2b, but not when either MO is injected at sub-phenotypic doses together with MO:control.(N) spec1 expression is lost in fgf8 morphants at the formative stages of MHB morphogenesis. (O) Putative model of the predicted functional pathways of fgf8/erk-mediated regulation of grhl2b in MHB maintenance, neural apoptosis and MHB folding. PHENOTYPE:
|
|
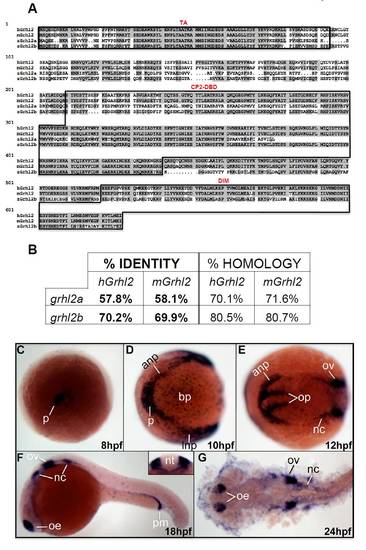
Comparison of human, mouse and zebrafish Grhl2 orthologs. (A) Amino acid alignment of Grhl2 from human (hGrhl2), mouse (mGrhl2) and zebrafish (zGrhl2a;zGrhl2b); boxed regions highlight highly conserved functional domains (TA, transactivation domain; DBD, DNA-binding domain; DIM, dimerisation domain). (B) Table showing the percentage identity and homology between human, mouse and zebrafish grhl2a and grhl2b sequences at the amino acid level. (C-G) Analysis of zebrafish grhl2b mRNA expression by in situ hybridisation from 8 hpf to 24 hpf. p, polster; anp and lnp, anterior and lateral neural plate; bp, brain primordium; nc, neural crest, ov, presumptive otic vesicles; op, optic primordium; oe, olfactory epithelium; pm, pronephric mesoderm. Expression is seen in the neural crest, both adaxially and ventrally to the neural tube (inset, nt, sagittal view) and otic vesicles. Flat-mount, dorsal view at 24 hpf (G) shows persistent expression in the olfactory epithelium, otic vesicles and neural crest. EXPRESSION / LABELING:
|
|
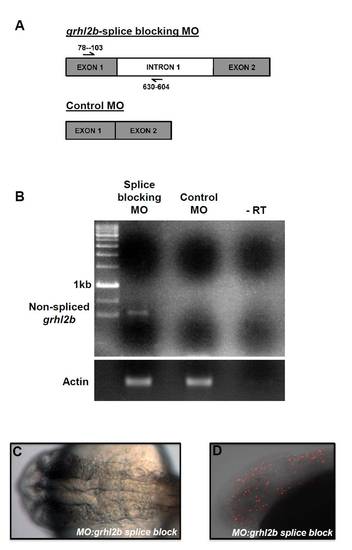
Generation, characterisation and effects of a grhl2b exon 1/intron 1 splice-blocking morpholino. (A) Schematic of PCR primers used to detect non-spliced reverse-transcribed mRNA product following block of splicing of grhl2b. (B) A specific grhl2b non-spliced product was detectable by RT-PCR; this product was absent in MO:control-injected fish. (C,D) The grhl2b splice-blocking MO reproduced the MHB-folding (C) and apoptosis (TUNEL; D) phenotype observed with the grhl2b ATG-blocking MO. The apoptosis in grhl2b morphants is most abundant in, but not restricted to, neural regions. PHENOTYPE:
|
|
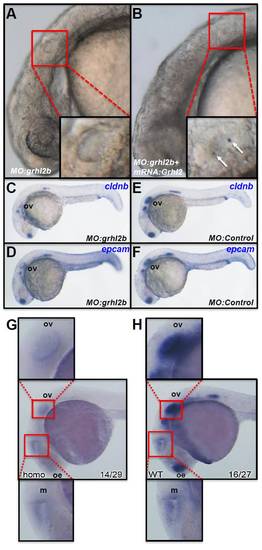
Otic vesicle defects following loss of grhl2b and persistence of grhl2b in mutant midbrain. (A,B) Loss of otoliths is visible in grhl2b morphants; these can be rescued by re-injection with Grhl2 mRNA (arrows; B). (C-F) Fish injected with the grhl2b-ATG MO show loss of otic cldnb (C) and epcam (D) expression, relative to controls (E,F). (G,H) Although expression in the olfactory epithelium (oe) and otic vesicle (ov) is greatly reduced in homozygous-null grhl2b mutants (homo; G), expression of grhl2b in the midbrain (m) is only slightly reduced compared with wild-type fish (WT; H). PHENOTYPE:
|
|
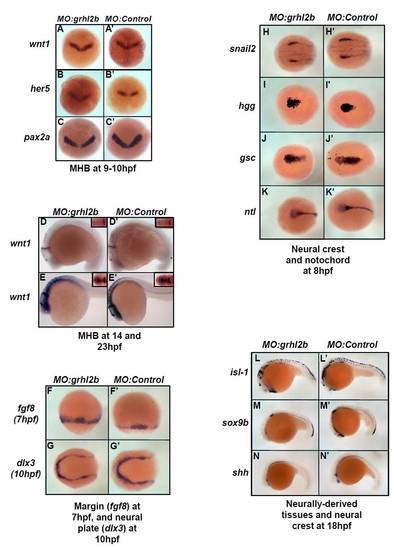
Expression of non-MHB developmental markers is unaffected in grhl2b-morphants. (A-N′) grhl2b morphants were examined for potential differences in expression of markers of the MHB at 9-10 hpf (A,A′, wnt1; B,B′, her5; C,C′, pax2a), 14 hpf (D,D′; wnt1, inset, dorsal view) and 23h pf (E-E2 wnt1, inset, dorsal view); margin and neural plate at 7-10h pf (F,F2, fgf8; G,G′, dlx3), neural crest at 8 hpf (H,H′, snail2), notochord at 8 hpf I,I2, hatching gland 1 (hgg); J,J′, goosecoid (gsc); K,K2 no tail (ntl) and ectoderm-derived tissues at 18 hpf, such as primitive neurons L,L′, islet 1 (isl1), neural crest (M,M′, sox9b) and floor plate of the neural tube N,N2, sonic hedgehog (shh). A-C show dorsal views, anterior towards top. D,E,L-N show lateral views, anterior towards left. F,H-K show dorsal views, anterior towards left. G shows a lateral view, anterior towards the top. Subtle differences in staining patterns were occasionally observed as a secondary, non-specific consequence of gross morphant morphology. |
|
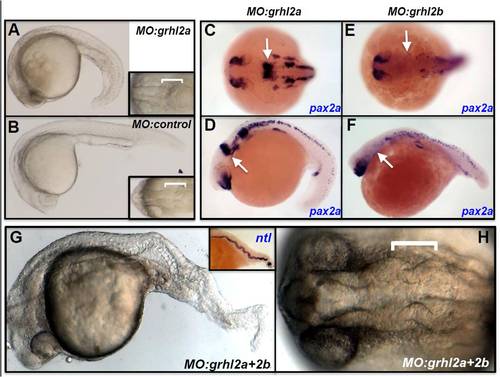
grhl2a is involved in axial, but not MHB, patterning. (A,B) MO-mediated knockdown of grhl2a leads to defects reminiscent of those in mutants with failed convergence-extension mediated movements, characterised by a shorter, broader trunk relative to controls. (C-F) The expression of MHB markers (arrows) is not lost following MO-mediated knockdown of grhl2a (pax2a; C-D), compared with the loss seen after MO-mediated knockdown of grhl2b (pax2a; E,F). (G,H) Following MO-mediated downregulation of both grhl2a and grhl2b, severe CE-like defects are seen in axial patterning, formation of the notochord (highlighted by brightfield microscopy and in situ hybridisation expression of the specific notochord marker, ntl; inset), with only minor defects in the formation of the MHB constriction (bracket; H). |
|
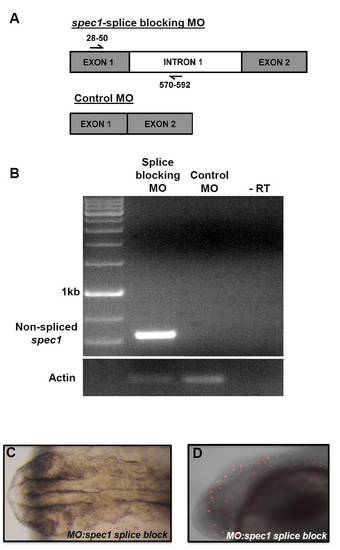
Generation, characterisation and effects of a spec1 exon 1/intron 1 splice-blocking morpholino. (A) Schematic of PCR-primers used to detect non-spliced reverse-transcribed mRNA product following block of splicing of spec1. (B) A specific spec1 non-spliced product was detectable by RT-PCR; this product was absent in MO:control-injected fish. (C,D) The spec1 splice-blocking MO reproduced the MHB-folding phenotype (C) observed with the spec1 ATG-blocking MO, although less apoptosis was visible (TUNEL; D). PHENOTYPE:
|
|
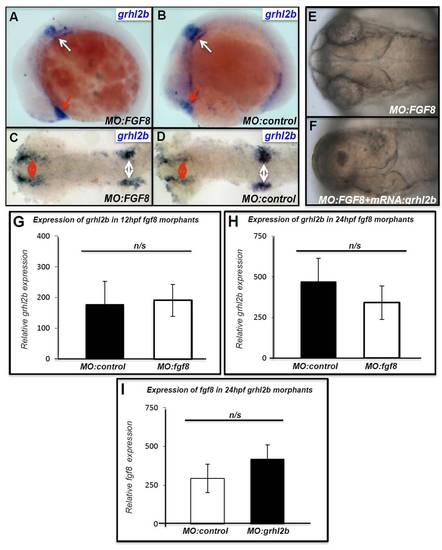
grhl2b mRNA fails to rescue the fgf8 MO-induced ace phenotype. (A-D) Expression of grhl2b in MO:fgf8-injected embryos at 14 hpf in lateral (A,B) or flatmount dorsal (C,D) views. Red arrows, olfactory placode; white arrows otic vesicles and lateral neural crest. No significant differences are seen in the abundance or intensity of grhl2b staining. (E,F) The ace phenotype induced by the fgf8 MO in the absence (E) and presence (F) of grhl2b mRNA. (G,H) Q-RT-PCR shows that expression of grhl2b in the MHB region of fgf8-morphants at 18 hpf (G) or 24 hpf (H) is unchanged. (I) Q-RT-PCR shows that expression of fgf8 in the MHB region of grhl2-morphants at 24 hpf is unchanged. |