- Title
-
Partially redundant proneural function reveals the importance of timing during zebrafish olfactory neurogenesis
- Authors
- Madelaine, R., Garric, L., and Blader, P.
- Source
- Full text @ Development
|
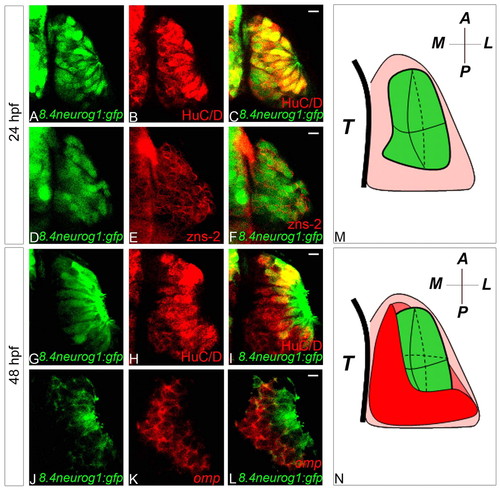
Tg(8.4neurog1:gfp) is a marker of zebrafish early-born olfactory neurons. (A-F) Single confocal sections of olfactory placodes showing co-expression of HuC/D (A-C) or the previously described pioneer neuron marker zns-2 (D-F) with GFP from the Tg(8.4neurog1:GFP) transgene at 24 hpf. (G-L) Single confocal sections of olfactory placodes showing immunolabelling of HuC/D (G-I) or in situ hybridisation and immunolabelling of omp (J-L) and GFP from the Tg(8.4neurog1:GFP) transgene at 48 hpf. Whereas all GFP-positive cells in the placode are HuC/D- and zns-2-positive at 24 hpf, at 48 hpf HuC/D+/GFP–cells are present; little, if any, overlap is detected between the expression of omp transcripts and GFP at 48 hpf. (M,N) Schematics of olfactory placodes at 24 hpf (M) or 48 hpf (N). Early-born olfactory neurons are shown in green and olfactory sensory neurons in red. A, anterior; L, lateral; M, medial; P, posterior; T, telencephalon. Scale bars: 10 μm. |
|
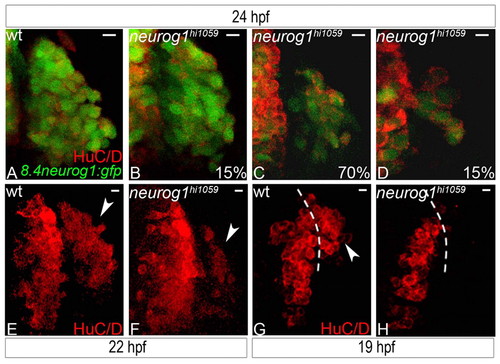
Zebrafish early-born olfactory neuron development is delayed in the absence of neurog1 function. (A-D) Confocal projections of wild-type (A) or neurog1 mutant (B-D) olfactory placodes at 24 hpf labelled for HuC/D and GFP from the Tg(8.4neurog1:GFP) transgene; mutant embryos show a variable but reduced number (shown as percentage of wild type) of early-born olfactory neurons. (E,F) Confocal projections of wild-type (E) or neurog1 mutant (F) olfactory placodes at 22 hpf labelled for HuC/D; a reduced number of HuC/D-positive neurons is detected in the olfactory placode of mutant embryos at this stage. (G,H) Confocal projections of wild-type (G) or neurog1 mutant (H) olfactory placodes at 19 hpf labelled for HuC/D; a complete absence of HuC/D neurons is apparent in the olfactory anlagen in neurog1 mutant embryos. Dashed white line indicates the boundary of the telencephalon and the olfactory placode; arrowheads indicate HuC/D expression in olfactory placode. Scale bars: 10 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
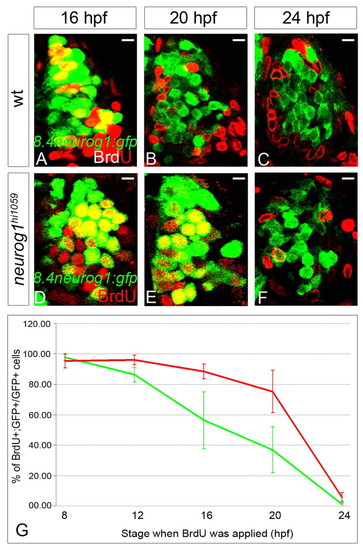
The last S-phase of early-born olfactory neurons is delayed in the neurog1 mutant zebrafish. (A-F) Confocal sections of olfactory placodes immunolabelled for BrdU (red) and GFP (green) from the Tg(8.4neurog1:GFP) transgene in wild-type (A-C) or neurog1 mutant (D-F) embryos at 24 hpf (A,B,D,E) or 28 hpf (C,F). The stages at which BrdU incubation were started is indicated. Scale bars: 10 μM. (G) The proportion BrdU+;GFP+ cells over total number of GFP+ cells in the olfactory placode after BrdU treatment in wild-type (green line) or neurog1 mutant (red line) embryos. Although early-born olfactory neurons generally leave the cell cycle later in mutant than in wild-type embryos, at 24 hpf few, if any, early-born olfactory neurons are still cycling in either genetic context. Error bars represent s.d. EXPRESSION / LABELING:
PHENOTYPE:
|
|
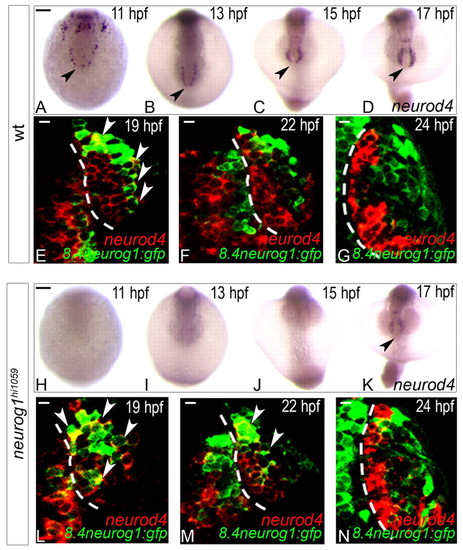
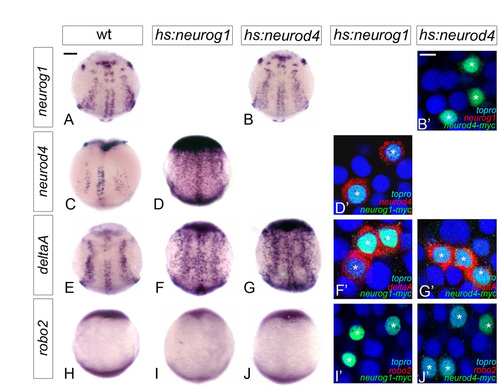
Expression of neurod4 in the zebrafish olfactory anlage is dependent on neurog1 at early but not late stages. (A-D,H-K) Whole-mount in situ hybridisation against neurod4 in wild-type (A-D) and neurog1 mutant (H-K) embryos at 11 (A,H), 13 (B,I), 15 (C,J) and 17 (D,K) hpf. Although neurod4 expression is absent in mutant embryos up to 15 hpf, expression recovers to wild-type levels at 17 hpf. Embryos are viewed dorsally with anterior down; black arrowheads indicate the mixed telencephalo-olfactory expression domain. (E-G,L-N) Single confocal sections of in situ hybridisation and immunolabelling against neurod4 and GFP from the Tg(8.4neurog1:GFP) transgene in the olfactory placode of wild-type (E-G) or neurog1 mutant (L-N) embryos at 19 (E,L), 22 (F,M) and 24 (G,N) hpf. At 24 hpf, neurod4 is restricted to cells of the olfactory placode immediately adjacent to the telencephalon and is excluded from Tg(8.4neurog1:gfp)-positive early-born olfactory neurons. White arrowheads indicate double-labelled cells. Dashed white line indicates the boundary of the telencephalon and the olfactory placode. Scale bars: in A, 100 μM for A-D; in H, 100 μM for H-K; in E-G,L-N, 10 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
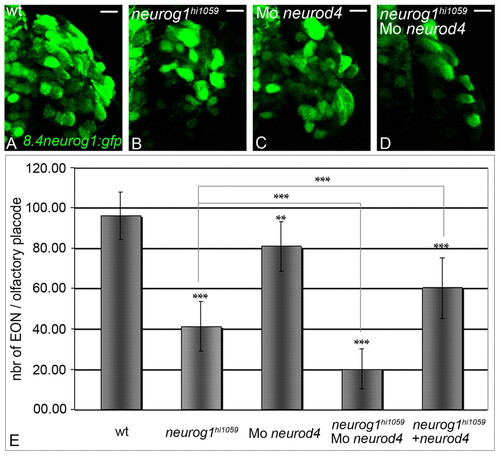
Neurog1 and Neurod4 act redundantly during development of zebrafish early-born olfactory neurons. (A-D) Confocal projections of GFP from the Tg(8.4neurog1:GFP) transgene in the olfactory placode of wild-type (A), neurog1 mutant (B), neurod4 morphant (C) and neurog1/neurod4 double loss-of-function (D) embryos at 24 hpf. Abrogation of both Neurod4 and Neurog1 function leads to a severe deficit in the differentiation of early-born olfactory neurons. Placodes are oriented with anterior up. Scale bars: 10 μM. (E) Counts of early-born olfactory neurons per placode at 24 hpf in wild-type, neurog1 mutant, neurod4 morphant, neurog1/neurod4 double loss-of-function embryos and neurog1 mutant embryos in which Neurod4 has been mis-expressed. A minimum of twelve olfactory placode was analysed for each context. Error bars represent s.d. *P<0.05, **P<0.001, ***P<0.0005, determined by t-test. |
|
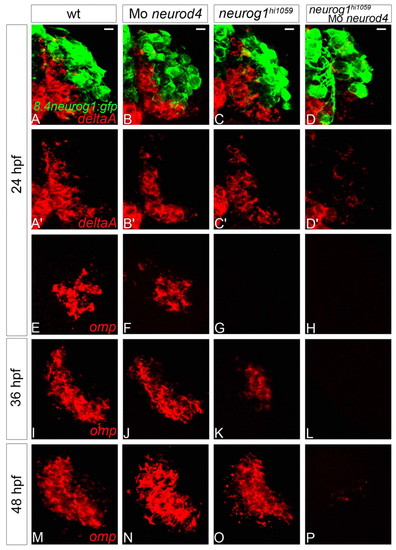
Neurod4 and Neurog1 act redundantly during OSN development in zebrafish. (A-D2) Confocal projections of in situ hybridisation and immunolabelling against deltaA and GFP in wild-type (A,A2), neurod4 morphant (B,B2), neurog1 mutant (C,C2) and neurog1/neurod4 double loss-of-function (D,D2) olfactory placodes of Tg(8.4neurog1:GFP) embryos at 24 hpf. Although no difference is apparent in the expression of deltaA in single loss-of-function embryos relative to wild-type siblings, deltaA expression is absent when both neurog1 and neurod4 function is abolished. (E-P) Confocal sections of in situ hybridisation labelling against olfactory marker protein (omp) in wild-type (E,I,M), neurod4 morphant (F,J,N), neurog1 mutant (G,K,O) and neurog1/neurod4 double loss-of-function (H,L,P) olfactory placodes at 24 (E-H), 36 (I-L) and 48 (M-P) hpf. Although omp expression resembles the wild-type pattern in the single loss-of-function context at 48 hpf, there is a delay in the initiation of omp expression in the absence of Neurog1 function. The expression of omp is never detected when both neurog1 and neurod4 function is abolished. Scale bars: 10 μm. |
|
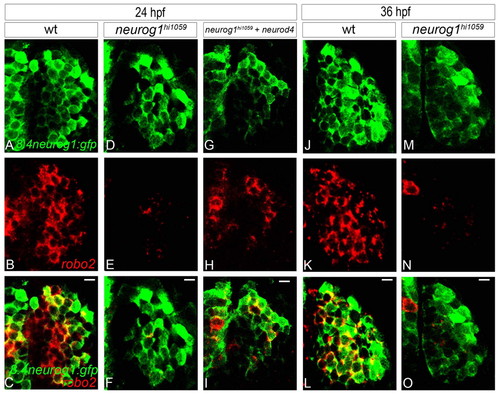
In neurog1 mutant zebrafish embryos, residual early-born olfactory neurons fail to express certain EON markers. (A-I) Confocal sections of in situ hybridisation and immunolabelling against robo2 and GFP from the Tg(8.4neurog1:GFP) transgene at 24 hpf in wild type (A-C), neurog1 mutant embryos (D-F) and neurog1 mutant embryos in which Neurod4 has been mis-expressed (G-I). Whereas early-born olfactory neurons in wild-type embryos robustly express robo2, expression is absent in neurog1 mutant embryos; mis-expression of Neurod4 partially rescues robo2 expression. (J-O) Confocal sections of in situ hybridisation and immunolabelling against robo2 and GFP from the Tg(8.4neurog1:GFP) transgene in wild-type (J-L) and neurog1 mutant (M-O) embryos at 36 hpf. The expression of robo2 never recovers in the few early-born olfactory neurons that develop in neurog1 mutant embryos. Scale bars: 10 μm. |
|
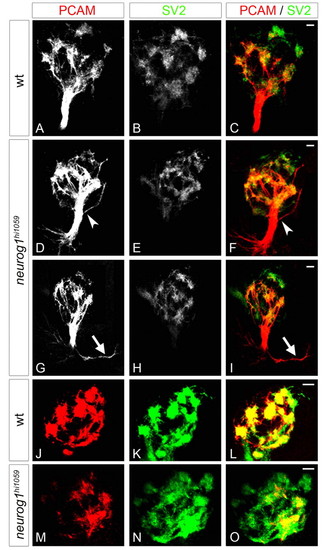
The fasciculation of olfactory axons and organisation of proto-glomeruli are flawed in neurog1 mutant zebrafish embryos. (A-I) Confocal projections of immunolabelling against PCAM and SV2 of olfactory projections in wild-type (A-C) and neurog1 mutant (D-I) embryos at 72 hpf. Whereas projections in wild-type embryos form a single fasciculated tract from the olfactory placode to the olfactory bulb, fasciculation (arrowheads in D,F) is aberrant in neurog1 mutant embryos; certain mutant embryos display abnormal guidance of projections immediately after they leave the placode (arrows in G,I). (J-O) Confocal projections of immunolabelling against PCAM and SV2 in the olfactory bulb in wild-type (J-L) and neurog1 mutant (M-O) embryos at 72 hpf. Proto-glomeruli appear less well organised in neurog1 mutant embryos relative to wild-type siblings. Embryos are viewed frontally (A-I) or dorsally with anterior up (J-O). Scale bars: 10 μM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
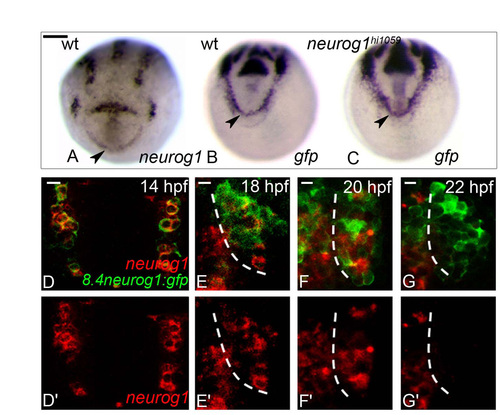
GFP expression from Tg(8.4neurog1:gfp) acts as a short term lineage label for endogenous neurog1-expressing cells. (A-C) Whole-mount in situ hybridisation against neurog1 in wild-type (A) or gfp from the Tg(8.4neurog1:GFP) transgene in wild-type (B) and neurog1 mutant embryos (C) at 10 hpf. Embryos are viewed dorsally with anterior down. At 10 hpf, neurog1 is expressed at the anterior border of the neural plate (A). In neurog1 mutant embryos, gfp+ cells are not lost in this territory (B versus C). Scale bar: 100 μM for A-C. (D-G2) Confocal sections of in situ hybridisation and immunolabelling against neurog1 and GFP in wild-type olfactory placodes in Tg(8.4neurog1:GFP) embryos at 14 (D,D2), 18 (E,E2), 20 (F,F2) and 22 (G,G2) hpf. neurog1 expression disappears between 20 and 22 hpf. Placodes are oriented with anterior up and lateral to the right. Dashed white line indicates the boundary of the telencephalon and the olfactory placode (E-G2). Scale bars: 10 μM. |
|
Potential target genes of proneural factors behave identically after mis-expression of Neurog1 and Neurod4. (A-J2) Whole-mount in situ hybridisation against neurog1 (A-B2), neurod4 (C-D2), deltaA (E-G2) and robo2 (H-J2) in wild-type (A,C,E,H) and hsp70:myc-neurog1 (D,D2,F,F2,I,I2) and hsp70:myc-neurod4 (B,B2,G,G2,J,J2) injected embryos at 10 hpf; injected embryos were heat-shocked at 7 hpf for 1 hour and fixed at 10 hpf. Heat-shocked embryos display ectopic expression of deltaA (E-G) but not of robo2 (H-J). In addition, mis-expression of Neurog1 is able to activate neurod4 expression. However, mis-expression of Neurod4 is not able to activate ngn1 expression. Confocal sections of the neural plate at 10 hpf show that mosaic mis-expression of a Myc-tagged form of Neurog1 or Neurod4 (in green and marked with an asterisk) induces deltaA (in red in F2,G2) in a cell-autonomous manner but not robo2 (I2,J2). However, whereas Myc-tagged Neurog1 induces cell-autonomous expression of neurod4 (in red in D2), Myc-tagged Neurod4 cannot induce neurog1 under similar conditions (Bý). Embryos are viewed dorsally with anterior up. Scale bars: in A, 100 μM for A-J; in B2, 100 μM for B2,D2,F2,G2,I2,J2. |
|
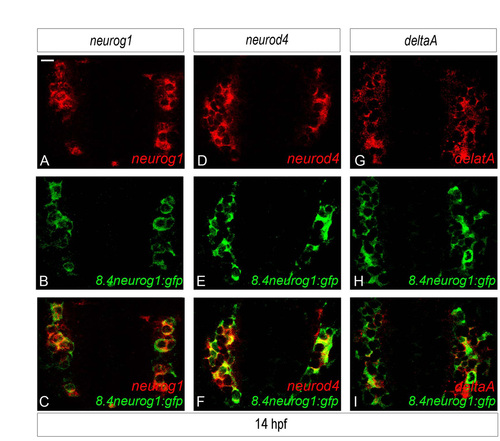
The expression of neurog1, neurod4 and deltaA overlap extensively in the anlage of the zebrafish olfactory placode. (A-I) Confocal sections of in situ hybridisation and immunolabelling against neurog1 (A-C), neurod4 (D-F) or deltaA (G-I) and GFP in Tg(8.4neurog1:GFP) embryos at 14 hpf. At this stage, neurog1 and it target genes are expressed in virtually all GFP+ cells, strongly suggesting that the three genes are co-expressed at this stage. Embryos are viewed dorsally and with anterior down. Scale bar: 10 μM for A-I. |
|
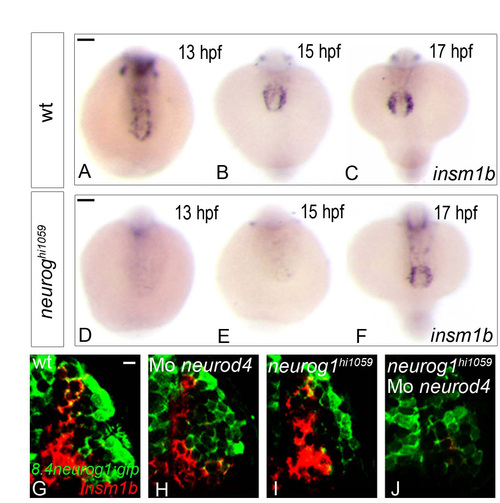
insm1b behaves as a proneural target in the developing zebrafish olfactory system. (A-F) Whole-mount in situ hybridisation against insm1b in wild-type (A-C) and neurog1 mutant (D-F) zebrafish embryos at 13 (A,D), 15 (B,E) or 17 (C,F) hpf. Although insm1b expression is absent in embryos up to 15 hpf in the mutant, its expression recovers at 17 hpf. Embryos are viewed dorsally with anterior down (D-F). (G-J) Confocal projections of in situ hybridisation and immunolabelling against insm1b and GFP in the olfactory placodes of wild-type (G), neurod4 morphant (H), neurog1 mutant (I) and neurog1/neurod4 double loss of function (J) embryos in Tg(8.4neurog1:GFP) embryos at 24 hpf. At this stage, insm1b+ cells are lost only in the neurog1/neurod4 double loss of function situation (J) suggesting that these two proneural factors are redundantly required in olfactory sensory neuron progenitors. Placodes are oriented with anterior up and lateral to the right. Scale bars: in A, 100 μM for A-C; in D, 100 μM for D-F; in G, 10 μM for G-J. EXPRESSION / LABELING:
|
|
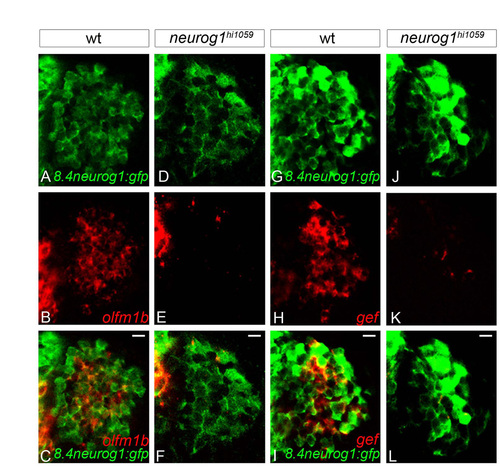
Residual early-born olfactory neurons fail to express olfm1a and gef in neurog1 mutant olfactory placodes. (A-L) Confocal sections of in situ hybridisation and immunolabelling against olfactomedin1b (olfm1b, A-F) or gefiltin (gef, G-L) and GFP in wild-type (A-C,G-I) and neurog1 mutant embryos (D-F,J-L) in the Tg(8.4neurog1:GFP) background at 24 hpf. Whereas early-born olfactory neurons differentiate in neurog1 mutant embryos, they do not express these markers of specification. Placodes are oriented with anterior up and lateral to the right. Scale bars: 10 μM. |
|
The phenotype of olfactory projections in neurog1 mutant embryos is generally stronger than that of robo2 mutants. (A-L) Confocal projections of PCAM/SV2-labelled olfactory projections in wild-type (A-C,G-I) and neurog1 mutant (D-F,J-L) embryos at 72 hpf. Whereas projections in wild-type embryos form a single fasciculated tract from the olfactory placode to the olfactory bulb, in certain neurog1 mutants a large number of projections are aberrantly oriented (arrowheads in D,F) or a portion of olfactory projections never seems to leave the olfactory placode (arrowheads in J,L). Embryos are viewed frontally (A-F) or dorsally with anterior up (G-L). Dashed white lines indicate the boundary of the telencephalon and the olfactory placode (J,L). Scale bars: 10 μM. |