- Title
-
Subnuclear development of the zebrafish habenular nuclei requires ER translocon function
- Authors
- Doll, C.A., Burkart, J.T., Hope, K.D., Halpern, M.E., and Gamse, J.T.
- Source
- Full text @ Dev. Biol.
|
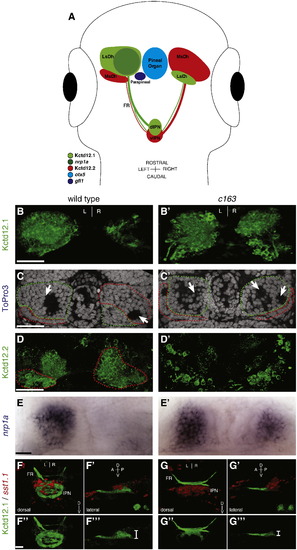
The lateral subnuclei of the habenulae are enlarged in c163 mutants. (A) Diagram depicting the relative expression patterns of genes in the epithalamus. (B) Kctd12.1 protein is detected in more cells of the left LsDh of the dorsal habenula than the right. (B′) By contrast, c163 mutants exhibit more Kctd12.1 neurons in both habenulae, particularly the right. (C) In wild-type larvae, the LsDh (green dashed line) includes a large, round soma-free area on the left but a smaller soma-free area on the right (white arrows). (C′) In c163 mutants, both the left and right habenula contain large cell-free areas (two are seen in the right habenula of this example). (D) In WT larvae, neurons of the MsDh express Kctd12.2, and are more abundant in the right dorsal habenula than the left. The soma of the Kctd12.2+ neurons are outlined in red (based on nuclear staining by ToPro3 [not shown]). Kctd12.2 expression is also detected in the extensive dendritic branches of MsDh neurons in the left habenula. (D′) In the dorsal habenulae of c163 mutants, the number of cells expressing Kctd12.2 is reduced. (E) nrp1a is expressed in a lateral portion of the LsDh of wild type embryos. (E′) nrp1a expression is expanded in the right habenula of c163 mutants. (F–F′′′) Axons from neurons expressing Kctd12.1 (green) target neurons of the dorsal and ventral IPN, which express somatostatin1.1 (sst1.1; red in F,F′) in WT embryos. (G–G′′′) In c163 mutants, Kctd12.1-expressing projections are confined to the ventraldomain of the IPN (white bracket: G′′′). All images are dorsal views at 4 dpf, except F′, F′′′,G′ and G′′′, which are lateral views. B, D, and F are 3D reconstructions of confocal z-stacks. C, C′ are single z-plane images from confocal z-stacks. LsDh: lateral subnucleus of the dorsal habenula. MsDh: medial subnucleus of the dorsal habenula. FR: fasciculus retroflexus. IPN: interpeduncular nucleus. Scale bars: (B–D = 30 μm; E = 10 μm; F′′ = 20 μm). |
|
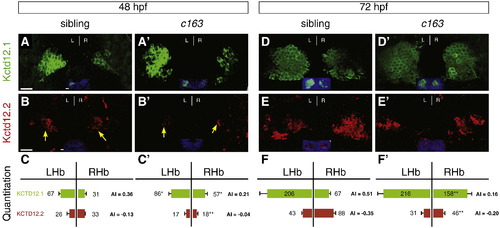
Kctd12.1 expression is significantly expanded and Kctd12.2 expression significantly decreased throughout larval development in c163 mutants. (A, B, C) In wild-type larvae at 48 hpf, there are 2.1 times as many Kctd12.1-expressing neurons (green) in the left LsDh of the dorsal habenula as in the right. In the MsDh, there are 1.3 times as many Kctd12.2-expressing neurons (red) in the right side as the left. (A′, B′, C′) In c163 mutants, more Kctd12.1+ and fewer Kctd12.2+ neurons are found in the right habenula. (D–F′) At 72 hpf, a similar situation to 48 hpf is observed. Images are 3D reconstructions of 50 μm-thick confocal Z-stacks through the epithalamus. Scale bars represent 10 μm. Significance determined by Student′s T-test (*p < 0.05, **p < 0.01); n > 6. Insets in A–B′, D–E′ show ToPro3 labeling in a single confocal section of the Z-stack. EXPRESSION / LABELING:
PHENOTYPE:
|
|
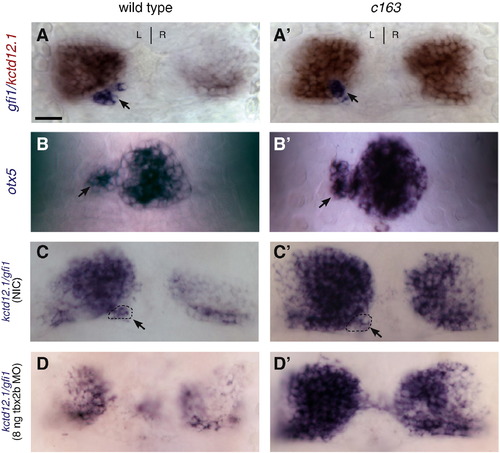
The enlarged LsDh phenotype of sec61al1c163 is independent of the parapineal organ. (A) In wild-type larvae, the parapineal organ (gfi-1, blue) is found adjacent to the left LsDh (kctd12.1, red). (A′) Likewise, sec61al1c163 mutants possess a single parapineal organ on the left side of the brain. (B) The pineal complex (consisting of the pineal and parapineal, labeled by otx5) is similar in WT and (B′) sec61al1c163 mutant larvae. (C) Compared to an non-injected control (NIC), with a parapineal organ on the left side of the brain (dashed oval), (D) disruption of parapineal organ formation using a tbx2b antisense morpholino causes a reduction in the size of the left LsDh (kctd12.1). (C′, D′) However, in sec61al1c163 mutants, reduction of the parapineal organ has no effect on the size of the LsDh on either the left or right side. All views are dorsal. Panels A, C, D = 96 hpf; B = 72 hpf. Parapineal indicated by black arrowheads A–C′. Scale bar = 10 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
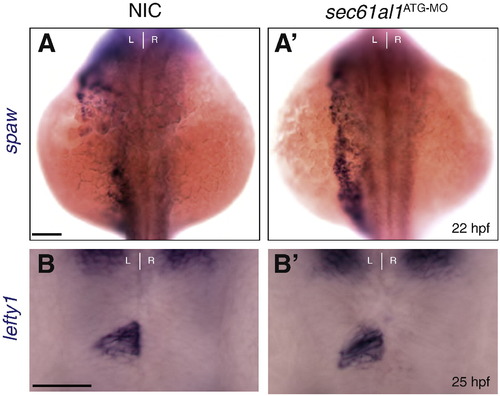
Depletion of sec61al1 does not affect expression of Nodal pathway genes in the left lateral plate mesoderm and epithalamus. (A) southpaw (spaw) is expressed in the left lateral plate mesoderm at 22 hpf in non-injected controls (NIC) (A′) as well as in sec61al1 morphants injected with 500 pgsec61al1ATG-MO (morphant: 15/16 left-side, 1/16 bilateral versus NIC: 9/11 left-side, 2/11 bilateral). (B) lefty1 expression is confined to the left side of the epithalamus at 25 hpf in NIC and (B′) sec61al1 morphants (NIC: 19/22 left-sided, 3/22 absent; morphant: 27/29 left-sided, 2/29 absent). All views are dorsal. Scale bar = 10 μm. EXPRESSION / LABELING:
|
|
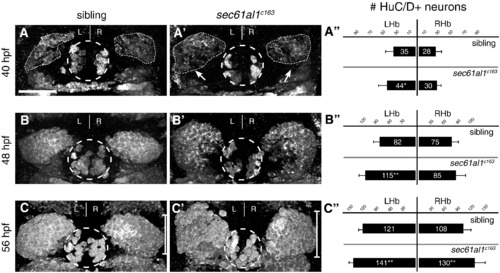
Excess habenular neurons form in sec61al1c163 mutant habenulae. (A–C′) Post-mitotic neurons in WT or sec61al1c163 mutant embryos as detected by HuC/D antibody labeling. (A) A representative sibling embryo has fewer HuC/D+ habenular neurons at 40 hpf as compared to (A′) a sec61al1c163 mutant. (A") Quantification of data at 40 hpf. The number of left habenula neurons was significantly increased in homozygous mutants compared to WT siblings. (B–B") At 48 hpf, significantly more neurons were detected in the left habenula of mutants than in WT controls. (C–C′′) By 56 hpf, both the left and right habenulae of mutants contain more neurons than controls. In all panels, the pineal organ is outlined by a dashed circle. Scale bar equals 30 μm. Significance determined by Student′s T-Test (*p < 0.05, **p < 0.01); 40 hpf (n = 12), 48 hpf (n = 9), 56 hpf (n = 12). Images are extended focus projections of confocal Z-stacks through the dorsal diencephalon. |
|
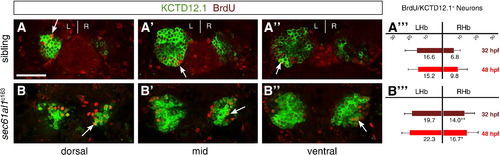
Increased cell division of LsDh precursors in sec61al1c163 mutants. Embryos were briefly pulsed with BrdU at 32 hpf and chased until 4 dpf, when they were labeled for Kctd12.1 (green) and BrdU (red). As neurons are post-mitotic, brightly labeled BrdU+ cells are those that underwent a final mitosis during the BrdU pulse (white arrows); see text for further explanation of the labeling strategy. (A–A′′′) Compared to WT controls, (B–B′′′) significantly more neurons are born in the RHb of sec61al1c163 mutants at 32 hpf. This trend continues through 48 hpf (A′′′,B′′′), as again there are significantly more neurons in the RHb of sec61al1c163 mutants (images not shown) (Andres et al., 1999). Images are single z-plane images from confocal z-stacks. Dorsal (A,B), mid (A′,B′), and ventral (A′′,B′′) sections are shown. Scale bar represents 30 μm. Significance determined by Student′s T-test (*p < 0.05; **p < 0.01); n = 41 siblings, n = 43 mutants. |
|
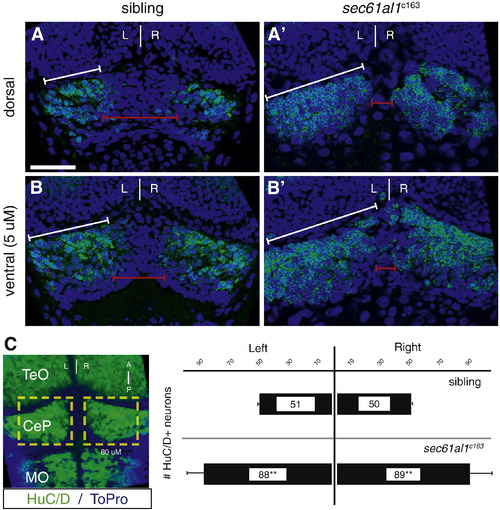
Excess neurons are found in the cerebellum of sec61al1c163 mutants. We quantified neurons in the dorsal-most region of the cerebellum as a comparison to the development of the dorsally-located habenulae. HuC+ neurons are evident in optical sections from the (A) dorsal surface or (B) 5 μm below the dorsal surface of the cerebellar plate (white brackets) at 72 hpf. (A′, B′) More HuC+ cerebellar neurons are evident in sec61al1c163 mutants, particularly in the medial region of the cerebellar plate (red brackets). (C) Representative extended focus image of the hindbrain for orientation; graphs represent data from 5 larvae. Images in A–B′ are single plane images from confocal Z-stacks, with B,B′ taken 5 μm ventral to A,A′, respectively. Image C is an extended focus projection of a confocal Z-stack. Significance determined by Student′s T-test, comparing the left, right, or total cerebellar neurons of sec61al1c163 mutants with siblings (p = 0.0002, 0.002, 0.0005, respectively); n = 5, mutants and siblings. TeO = optic tectum; CeP = cerebellar plate; MO = medulla oblongata. Scale bars = 30 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
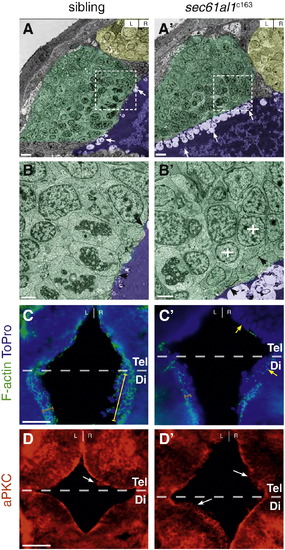
The ventricular epithelium is disrupted in sec61al1c163 mutants. (A) Transmission electron micrographs from coronal sections through the dorsal diencephalon of WT and (A′) sec61al1c163 mutant larvae at 54 hpf, with the pineal organ (yellow), habenulae (green), and 3rd ventricle (purple) indicated by false coloring. (B, B′) Higher magnification shows significantly more apical blebbing from cells lining the ventricle in mutants compared to their siblings (arrows). (B) The well-defined columnar morphology and cell–cell junctions (black arrowheads) of WT ventricular cells are not found (B′) in mutants. Rather, cells proximal to the ventricle are rounded (white crosses) and have few cell–cell junctions (black arrowheads). (C) Filamentous actin (F-actin) in the ventricular neuroepithelium localizes to the apical membrane (orange bracket) in cells along the entire rostro-caudal axis of the anterior 3rd ventricle. (C′) sec61al1c163 mutants have reduced (orange bracket) F-actin in the caudal ventricular neuroepithelium (orange bracket) and many cells without apically-localized F-actin in the rostral ventricular neuroepithelium (yellow arrowheads). (D) aPKC localizes to the apical surface of the ventricular neuroepithelium in wild type embryos, whereas (D′) apical aPKC is reduced or absent in many cells of mutants. C, D are from 10 μm thick cryosections, taken from the dorsal aspect. F-actin sections (C) are roughly 30 μm ventral from the dorsal surface, and aPKC sections (D) roughly 10 μm from the dorsal diencephalon. Tel, telencephalon; Di, diencephalon. Dashed boxes in A, A′ correspond to the region of higher magnification in the adjacent micrographs, B, B′. Scale bars: A,A′ = 20 7mu;m; B,B′ = 5 μm; C–D′ = 20 μm. |
|
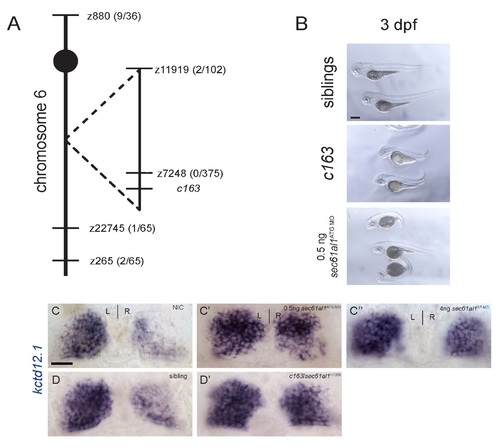
The c163 lesion is a nonsense mutation in sec61al1. (A) Linkage to microsatellite markers was used to map c163 close to z7248, near the sec61al1 locus. (B) c163 mutants are characterized by a reduction in jaw and head volume, and a short, dorsally curved axis. These phenotypes are also seen in embryos injected with 500 pg sec61al1ATG-MO. (C) Compared to a non-injected control, (C′) treatment with a morpholino complementary to the start site or (C′′) to a splice acceptor site in the sec61al1 transcript results in excess kctd12.1-expressing neurons in the right habenula. (D, D′) The retroviral insertion mutant, sec61al1hi1058 fails to complement c163. Transheterozygotes for hi1058 and c163 have increased numbers of kctd12.1-expressing cells in the habenulae. Scale bars: B-D′ = 20 µm. PHENOTYPE:
|
|
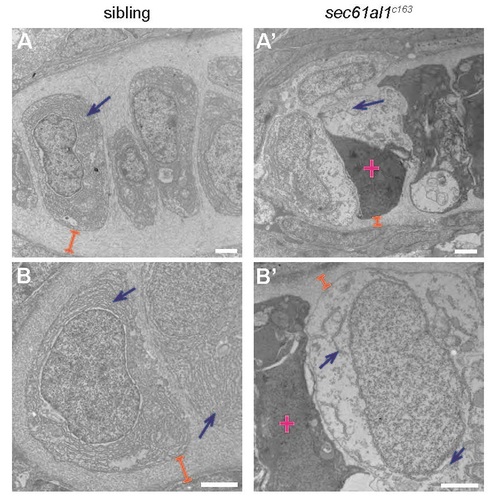
Transmission electron micrographs of jaw chondrocytes show ER and ECM deficits in sec61al1c163 mutants. (A, B) Chondrocytes of WT controls have dense ER throughout the cytoplasm (blue arrows) and extensive ECM surrounding chondrocytes (orange brackets). (A′, B′) Endoplasmic reticuli are sparse and discontinuous in sec61al1c163 mutants and the ECM is reduced in thickness. Dead cells are common in sec61al1c163 mutant micrographs (pink cross). All scale bars = 2 µm. |
|
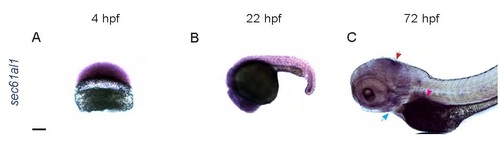
sec61al1 is maternally provided and extensively expressed in the embryo and larva. (A) Maternally provided sec61al1 mRNA (purple) in the blastoderm at 4 hpf. (B) Extensive expression of sec61al1 at 22 hpf, throughout the head and trunk. (C) By 72 hpf, sec61al1 transcript is expressed throughout the head, and more highly expressed in the jaw (blue arrow), midbrain/hindbrain boundary (red arrow), and exocrine pancreas (pink arrow). Scale bars = 10 µm. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 360(1), Doll, C.A., Burkart, J.T., Hope, K.D., Halpern, M.E., and Gamse, J.T., Subnuclear development of the zebrafish habenular nuclei requires ER translocon function, 44-57, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.