- Title
-
Conserved Regulation of p53 Network Dosage by MicroRNA-125b Occurs through Evolving miRNA-Target Gene Pairs
- Authors
- Le, M.T., Shyh-Chang, N., Khaw, S.L., Chin, L., Teh, C., Tay, J., O'Day, E., Korzh, V., Yang, H., Lal, A., Lieberman, J., Lodish, H.F., and Lim, B.
- Source
- Full text @ PLoS Genet.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
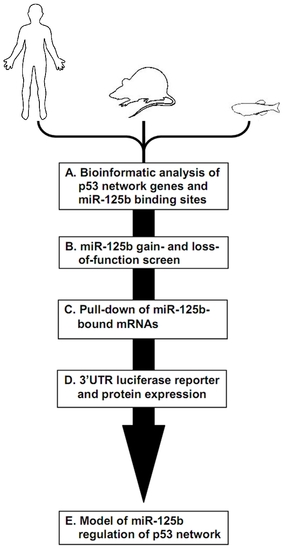
Schematic of experimental design and workflow. (A) Bioinformatic analysis was performed on p53 network genes listed in the Ingenuity Pathways Analysis database and p53 Knowledgebase, and miR-125b binding sites predicted by the TargetScan and MicroCosm databases. (B) p53 network genes were screened for miR-125b targets by using gain- (GOF) and loss-of-function (LOF) of miR-125b in human cells, mouse cells and zebrafish embryos, as indicated by effects on gene expression using qRT-PCR. (C) p53 network genes that were positive in either the GOF or LOF screen were assayed for direct binding to miR-125b using a biotinylated microRNA pull-down method. (D) p53 network genes that were also positive in the miR-125b pull-down were finally validated as miR-125b targets by 3′ UTR luciferase reporter assays and Western blots for protein expression. (E) A model of how miR-125b regulates the p53 network across vertebrates was constructed using our combined datasets for human, mouse and zebrafish cells. |
|
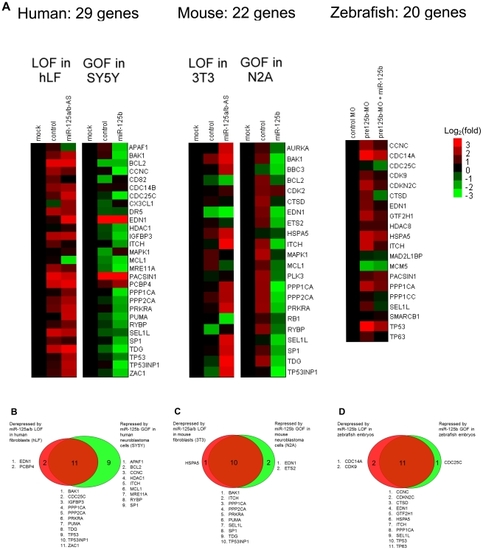
(A) Loss-of-function (LOF) screens were performed in human primary lung fibroblasts (hLF) or mouse 3T3 fibroblasts by transfecting an antisense RNA against both miR-125a and miR-125b (miR-125a/b-AS), or by microinjecting morpholinos (MO) against pre- |
|
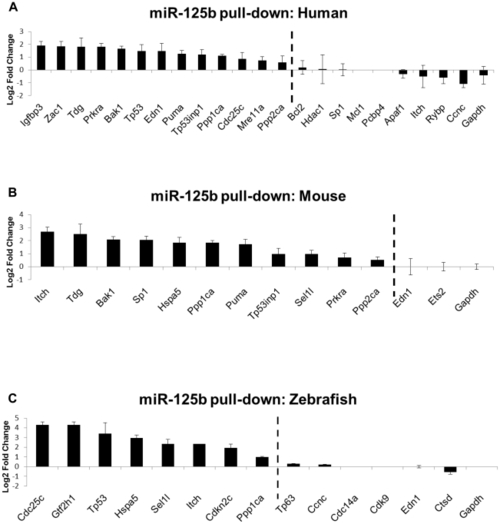
Biotinylated miR-125b was used as bait to pull-down mRNAs bound to miR-125b, using streptavidin-conjugated magnetic beads. The mRNAs were quantified by qRT-PCR, normalized to |
|
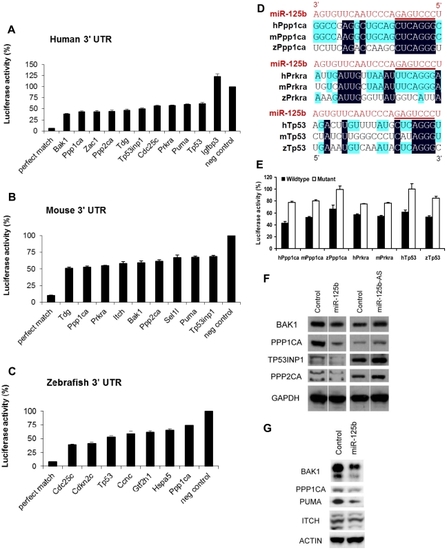
Candidate p53 network genes that were positive in both the GOF/LOF screen and miR-125b pull-down were validated for targeting by miR-125b using the 3′ UTR luciferase reporter assay and Western blots for protein expression. (A-C), Reporter genes containing the full-length 3′ UTRs of each selected target gene were co-transfected with miR-125b duplex into 293T cells. Luciferase readings were obtained 48 hours after transfection and presented here as the average percentage of luciferase activity ± s.e.m. (n≥3) relative to a scrambled duplex co-transfected control (100%). A reporter containing a 23-nucleotide-binding-site with perfect complementarity to miR-125b was used as the perfect match positive control, while the unmodified luciferase reporter was used as the empty negative control. (A) Human: 10 out of 13 candidate genes' 3′ UTRs showed significant repression by miR-125b relative to the control (p<0.01). (B) Mouse: 9 out of 11 candidate genes' 3′ UTRs showed significant repression by miR-125b relative to the control (p<0.01). (C) Zebrafish: 7 out of 8 candidate genes' 3′ UTRs showed significant repression by miR-125b relative to the control (p<0.01). (D) Alignment of predicted |
|
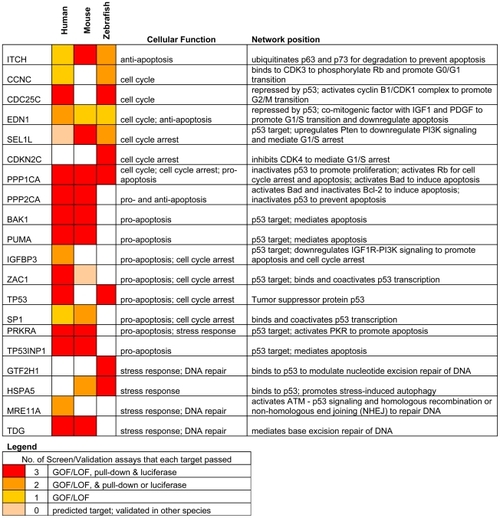
Only targets that passed ≥ 2 validation assays, in at least one species, are shown. Red: predicted targets validated by 3 assays; Orange: predicted targets validated by 2 assays; Yellow: predicted targets validated by 1 assay; Pink: predicted targets not validated by any assay, but validated by 3 assays in another species. |
|
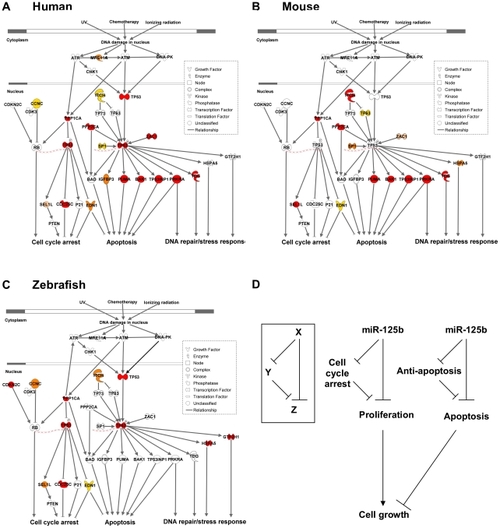
(A) Human p53 network. (B) Mouse p53 network. (C) Zebrafish p53 network. Models were constructed by Ingenuity Pathway Analysis. Red: predicted targets validated by 3 assays; Orange: predicted targets validated by 2 assays; Yellow: predicted targets validated by 1 assay; Pink: predicted targets not validated by any assay, but validated by 3 assays in another species. (D) Incoherent feedforward loop (FFL) motifs characterize miR-125b regulation of p53 network genes that mediate apoptosis or cell cycle arrest. |