- Title
-
Identification and mechanism of regulation of the zebrafish dorsal determinant
- Authors
- Lu, F.I., Thisse, C., and Thisse, B.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
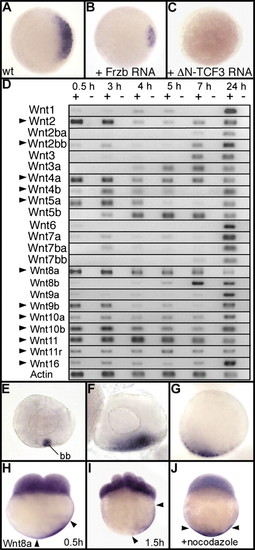
The secreted Wnt ligand Wnt8a is a strong candidate for the dorsal determinant in zebrafish. Expression of chordin at the sphere stage is shown in (A) wild type (WT), (B) an embryo injected with 500 pg of Frzb mRNA, and (C) an embryo injected with 100 pg of mRNA coding for a dominant-negative Tcf-3 (DN-Tcf3) (34). Embryos are in animal pole view with dorsal to the Right. (D) Expression of the 23 Wnt genes present in the zebrafish genome analyzed by RT-PCR at 1- to 2-cell stage (0.5 h), 1,000-cell stage (3 h), sphere stage (4 h), 40% epiboly (5 h), 60% epiboly (7 h), and at 24 hpf. Arrowheads indicate Wnt genes for which mRNAs are maternally deposited in the egg. (E–J) Whole mount in situ hybridization reveals that Wnt8a mRNAs accumulate in the Balbiani body (bb) in stage I oocytes (E) and are localized in a vegetal position in stage II (F) and stage III oocytes (G). Transcripts of Wnt8a are also observed in cleavage-stage embryos both in blastomeres and in the yolk cortical cytoplasm in vegetal position at (H) the 2-cell stage and (I) 16-cell stage. (J) Treatment with nocodazole, which depolymerizes microtubules, prevents transport of Wnt8a mRNA. Embryos are in lateral view with dorsal to the Right. Arrowheads in H–J indicate the limits of Wnt8a mRNA localization in the cortical cytoplasm. |
|
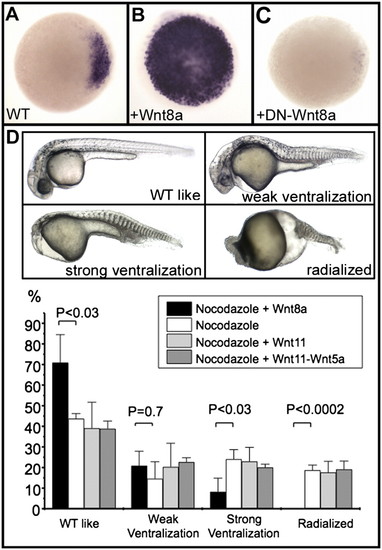
Wnt8a acts as the initial dorsal determinant in zebrafish. (A–C) Expression of chordin, analyzed by in situ hybridization, at the sphere stage in a wild-type (WT) embryo (A), in an embryo overexpressing Wnt8a (100 pg Wnt8a mRNA) (B), and in an embryo overexpressing a dominant-negative form of Wnt8a (500 pg of mRNA coding for DN-Wnt8a ORF2) (C). Embryos are in animal pole view, dorsal to the Right. (D) Nocodazole treatment generates embryos displaying various ventralization phenotypes ranging from weak (lacking notochord), strong (additionally missing the head), and radialized (lacking any dorsal territories). Injection of 25 pg of Wnt8a mRNA into one marginal blastomere at the 64-cell stage efficiently rescues radialized embryos and strongly reduces the frequency of strongly ventralized embryos; injections of 400 pg of Wnt11 mRNA or of a mix of 400 pg of Wnt11 mRNA and 400 pg of Wnt5a mRNA fails to rescue these phenotypes as shown in the graph. Embryos are presented in lateral view with anterior to the Left. Means and error bars (SD) were obtained from multiple experiments (three to five) with at least 50 embryos for each experimental condition. Comparisons between experiments were undertaken using unpaired Student′s t tests. P value of <0.05 was considered statistically significant. EXPRESSION / LABELING:
|
|
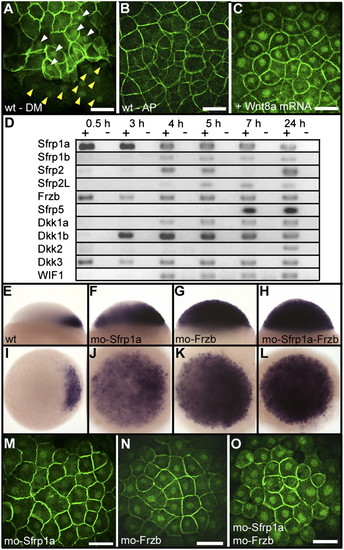
Negative regulation of the canonical Wnt pathway by Sfrp1a and Frzb. Immunofluorescence localization of β-catenin is shown at 3 hpf (high stage) (A), at the dorsal margin (DM), and (B) at the animal pole (AP) of a wild-type embryo or (C) at the animal pole of an embryo overexpressing Wnt8a (10 pg Wnt8a mRNA). White and yellow arrowheads in A point to the accumulation of β-catenin in blastomeres and yolk syncytial layer nuclei, respectively. (D) Analysis by RT-PCR of the expression of zebrafish Wnt inhibitors during early development. Sfrp (TLC), expressed only during gastrulation (35), is not presented here. Expression of chordin at the sphere stage in lateral view (Upper) and animal pole view (Lower) in (E and I) wild-type embryos, (F and J) Sfrp1a morphants, (G and K) Frzb morphants, and (H and L) Sfrp1a–Frzb double morphants. (M–O) Immunofluorescence localization of β-catenin in nuclei of the animal pole of embryos at the high stage in (J) Sfrp1a, (K) Frzb, and (L) Sfrp1a–Frzb morphants. Embryos are in dorsal view (A), lateral view (E–H), and in animal pole view (B, C, and I–O). (Scale bar, 100 μM.) |
|
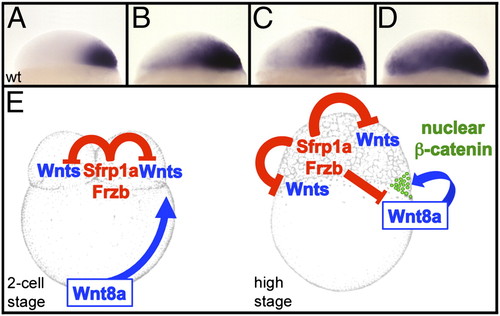
Sfrp1a and Frzb regulate the activation of the maternal Wnt/β-catenin signaling pathway. Expression pattern of chordin are shown in wild type (A) or in embryos injected with 4 ng (B), 5.5 ng (C), and 7 ng (D) of Frzb morpholinos. (E) At the two-cell stage Sfrp1a and Frzb prevent activation of the maternal Wnt pathway in the blastomeres, whereas the dorsal determinant, Wnt8a, is transported from the vegetal pole toward the blastomeres in a microtubule-dependent manner. At the high stage (immediately after the midblastula transition), Wnt8a translated from these transported mRNAs stimulates the canonical β-catenin pathway. Sfrp1a and Frzb are required to limit this activation to the cells receiving the highest level of stimulation. EXPRESSION / LABELING:
|
|
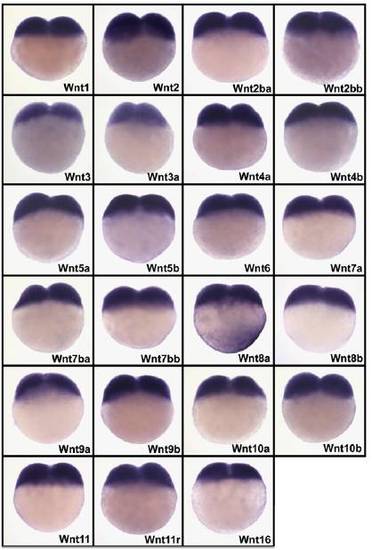
Analysis of spatial localization of mRNAs coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present in cortical cytoplasm of two-cell stage embryos. In situ hybridization was performed on wild-type embryos at the two-cell stage with specific antisense RNA probes corresponding to the different Wnts. The labeling reaction was performed for 24 h (10 times longer than usual) to detect possible low amounts of mRNA in the cortical cytoplasm. Due to strong overstaining, nonspecific labeling is observed in blastomeres for all Wnt probes. Only Wnt8a displays accumulation of mRNA in the cortical cytoplasm. |
|
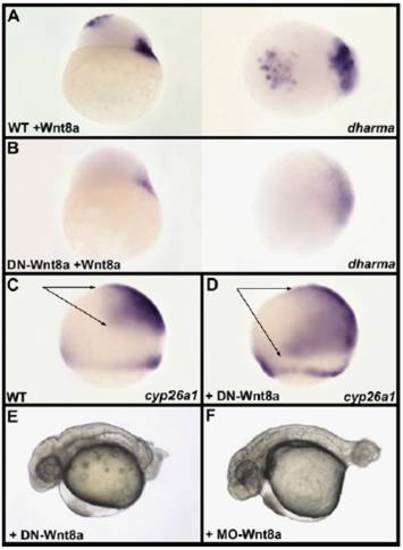
Antagonistic activity of the dominant-negative form of Wnt8a. (A) Injection of 10 pg of Wnt8a mRNA into one animal pole blastomere at the 64-cell stage of a wild-type embryo results in ectopic expression of dharma at the animal pole of a sphere-stage embryo. (Left) Lateral view. (Right) Animal pole view of the same embryo oriented dorsal to the Right. (B) Injection of 10 pg of Wnt8a mRNA in one animal pole blastomere at the 64-cell stage in an embryo previously injected at the one-cell stage with 500 pg of DN-Wnt8a mRNA does not result in ectopic expression of dharma. In addition, endogenous dharma expression at the margin is strongly attenuated. The antagonistic effect of DN-Wnt8a on the induction of dharma at the animal pole is higher than the induction of endogenous dharma because the injection of Wnt8a mRNA in animal pole blastomeres is performed 2 h after fertilization and 1.5 h after injection of DN-Wnt8a, whereas endogenous Wnt8a mRNA present in the cortical cytoplasm can be translated earlier. (Left) Lateral view. (Right) Animal pole view of the same embryo oriented dorsal to the Right. (C and D) Compared with its expression in wild type (WT) (C) the forebrain and midbrain expression of cyp26a1 (arrows) is strongly extended posteriorly (D) in embryos injected with 500 pg of DN-Wnt8a mRNA at the 8- to 16-cell stage. (E and F) At 30 hpf, embryo injected with 500 pg of DN-Wnt8a mRNA displays a phenotype (E) highly similar to the phenotype of Wnt8a morphant (F). Lu |
|
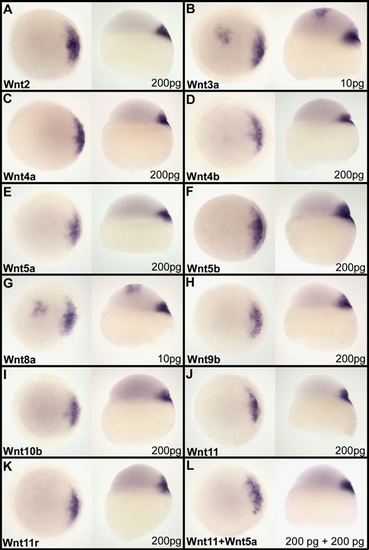
Assay of activation of the maternal β-catenin signaling pathway by canonical and noncanonical Wnt ligands. mRNA coding for canonical or noncanonical Wnts was injected together with GFP mRNA into one animal pole blastomere at the 64-cell stage. Embryos displaying a fluorescent clone at the animal pole were fixed at the sphere stage and the activation of the maternal β-catenin visualized by looking, by whole mount in situ hybridization, at the induction of ectopic expression of dharma at the animal pole. Wnt3a (B) and Wnt8a (G) are the only Wnts able to induce ectopic dharma expression. This is observed with as little as 10 pg of mRNA injected. All other Wnt ligands, canonical Wnts [Wnt2 (A), Wnt9b (H), and Wnt10b (I)], noncanonical Wnts [Wnt4a (C), Wnt4b (D), Wnt5a (E), Wnt5b (F), Wnt11 (J), and Wnt11r (K)], or the combination Wnt11–Wnt5a (L), which has been described to act as dorsal determinant in amphibian, are unable to efficiently activate the maternal β-catenin signaling pathway and fail to induce ectopic expression of dharma at the animal pole, even for 200 pg of mRNA injected. For each Wnt, assayed embryos are presented in both animal pole view (Left) and lateral view (Right). The amount of mRNA injected is indicated in the Lower Right corner. |
|
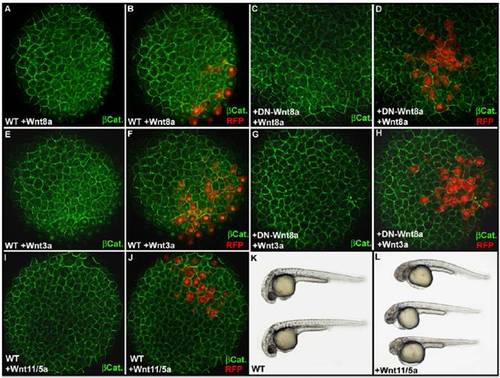
Effect of ectopic expression of Wnt8a, Wnt3a, and Wnt11/5a on the intracellular localization of β-catenin as revealed by immunofluorescence. Immunofluorescence localization of β-catenin at 3 hpf (high stage) at the animal pole of an embryo injected with 10 pg of Wnt8a (A) or Wnt3a (E) mRNA together with 50 pg of red fluorescent protein (RFP) mRNA in one animal pole blastomere at the 64-cell stage. (B and F) Overlay of the green channel, for visualization of β-catenin, and the red channel, for visualization of cells expressing the RFP and secreting Wnt8a or Wnt3a, reveals the activation of the maternal β-catenin signaling pathway (accumulation of β-catenin into the nucleus) at a distance up to three cell diameters from cells secreting Wnt8a (B) or Wnt3a (F). Injection of 500 pg DN-Wnt8a mRNA at the one-cell stage prevents stimulation of the maternal β-catenin signaling pathway by Wnt8a (C and D) or Wnt3a (G and H). (I and J) In the same experimental conditions, injection of a mix of 200 pg of Wnt11 mRNA and 200 pg of Wnt5a mRNA fails to activate the maternal β-catenin signaling pathway and does not induce nuclear accumulation of β-catenin in animal pole blastomeres within or at the vicinity of the Wnt11/Wnt5a secreting clone. (L) Injection of the same mix of 200 pg of Wnt11 mRNA and 200 pg of Wnt5a mRNA at the one-cell stage induces cyclopia phenotypes characteristic of overexpression of noncanonical Wnts, demonstrating that translation from these mRNAs produces active Wnt proteins. (K) Wild-type embryo. Embryos in A–J are in animal pole view. Embryos in K and L are at 30 hpf and are in lateral view anterior to the Left and dorsal to the Top. |
|
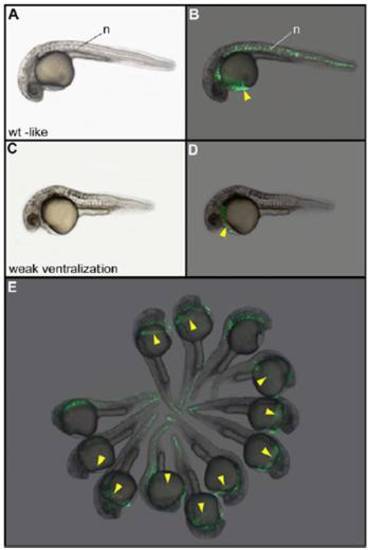
Phenotype and localization of the injected clone of cells in nocodazole-treated embryos injected with Wnt8a and GFP mRNAs. After treatment with nocodazole and injection of 25 pg of Wnt8a and 100 pg of GFP mRNAs into one blastomere at the 64-cell stage, embryos displaying a wild-type–like phenotype (A and B) always express the GFP in axial structures including the hatching gland (yellow arrowhead). Embryos displaying a weak ventralization phenotype (C and D) lack the notochord but always develop a hatching gland expressing GFP. (E) Field of embryos showing that the hatching gland is always derived from the injected blastomere. The two embryos lacking the hatching gland correspond to the class of strong ventralization phenotypes for which this tissue is not formed. (A, C, and E) Bright field. (B, D, and F) Localization of GFP fluorescent cells. n, notochord. Embryos are 30 h old and in lateral view. |
|
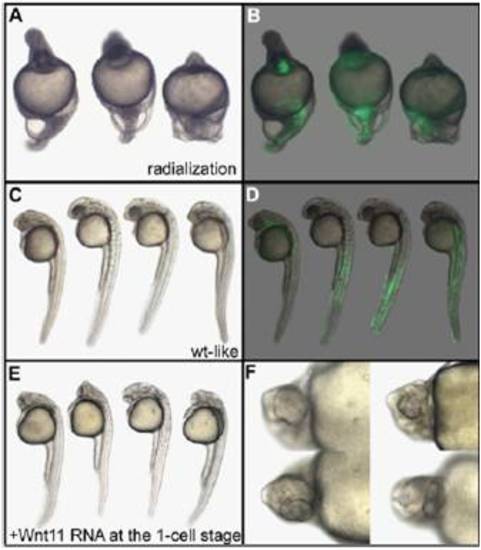
Phenotype and localization of the injected clone of cells in nocodazole-treated embryos injected with Wnt11 and GFP mRNAs. Embryos displaying a radialized phenotype after treatment with nocodazole and injection of 400 pg of Wnt11 and 100 pg of GFP mRNAs (A and B) demonstrate that Wnt11 is unable to rescue the lack of stimulation of blastomeres by dorsal determinants. Embryos displaying a wild-type–like phenotype (C and D) express the GFP in lateral and ventral (muscle and blood) but not in axial tissues (notochord and hatching gland). (E and F) Embryos injected with 400 pg of Wnt11 mRNA at the one-cell stage display cyclopia, demonstrating that the Wnt11 mRNA used is fully active. Embryos are 30 h old, anterior to the Top in lateral view (A–E), and seen in high magnification in ventral view (F). |
|
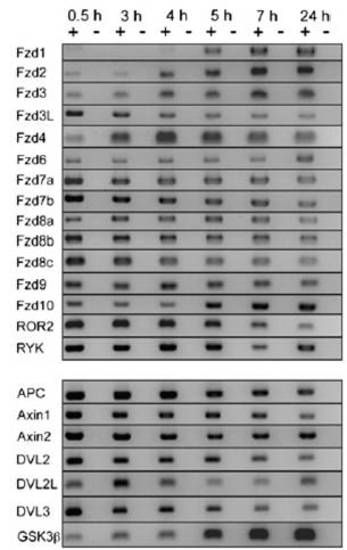
RT-PCR analysis of the expression of Wntreceptors and downstreamcomponents of the canonicalWntsignaling pathway. Expression of theWntreceptors (Fzd1, Fzd2, Fzd3, Fzd3L, Fzd4, Fzd6, Fzd7a, Fzd7b, Fzd8a, Fzd8b, Fzd9, Fzd10, ROR2, RYK) and of downstreamcomponents (APC, Axin1, Axin2, DVL2, DVL2L, DVL3, GSK3b) analyzed by RT-PCR at one- to two-cell stage (0.5 h), 1,000-cell stage (3 h), sphere stage (4 h), 40% epiboly (5 h), 60% epiboly (7 h), and at 24 hpf. |
|
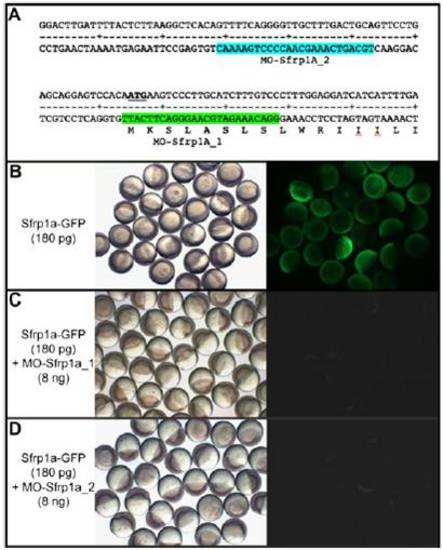
Specificity of Sfrp1a morpholinos. (A) Position of antisense morpholinos designed to block Sfrp1a translation on the sequence of 52-UTR and the 52 part of sfrp1a ORF. Injection of 180 pg of Sfrp1a-GFP mRNA at the one-cell stage results in embryos expressing the GFP at the late blastula stage (B). Coinjection of Sfrp1a-GFP mRNA with 8 ng of morpholino Sfrp1a_1 (C) or of Sfrp1a_2 (D) prevents translation of the Sfrp1a-GFP fusion protein. (Left) Bright field. (Right) Visualization of GFP fluorescence. |
|
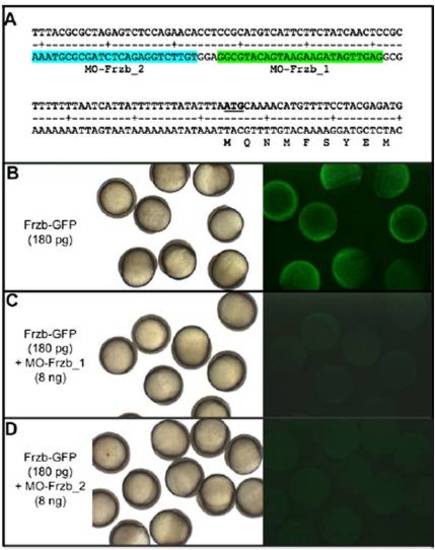
Specificity of Frzb morpholinos. (A) Position of antisense morpholinos designed to block Frzb translation on the sequence of 52-UTR and the 52 part of Frzb ORF. Injection of 180 pg of Frzb-GFP mRNA at the one-cell stage results in (B) embryos expressing GFP at the late blastula stage. Coinjection of Frzb-GFP mRNAs with 8 ng of morpholino Frzb_1 (C) or Frzb_2 (D) prevents translation of the Frzb-GFP fusion protein. (Left) Bright field. (Right) Visualization of GFP fluorescence. |