- Title
-
The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion
- Authors
- Wang, J., Panáková, D., Kikuchi, K., Holdway, J.E., Gemberling, M., Burris, J.S., Singh, S.P., Dickson, A.L., Lin, Y.F., Sabeh, M.K., Werdich, A.A., Yelon, D., MacRae, C.A., and Poss, K.D.
- Source
- Full text @ Development
|
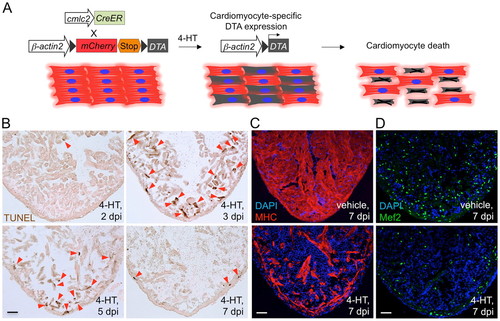
Genetic ablation of adult zebrafish cardiomyocytes. (A) Schematic representation of transgenes used for zebrafish cardiomyocyte ablation. DTA is specifically expressed in cardiomyocytes upon 4-HT treatment. (B) TUNEL staining of ventricular muscle from transgenic animals injected with vehicle or 4-HT (0.5 mg/ml). Arrowheads indicate TUNEL-positive cardiac myocytes. (C) Myosin heavy chain (MHC) staining of ventricular sections after vehicle or 4-HT injection, shown at 7 days post-injection (dpi). (D) Mef2 staining to indicate cardiomyocyte nuclei, showing major cellular losses in 4-HT-injected animals. Scale bars: 50 μm. |
|
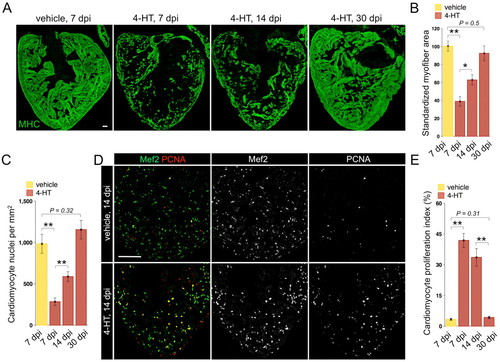
Rapid regeneration of ventricular cardiomyocytes after ablation-induced injuries. (A) Myosin heavy chain (MHC) staining of ventricular sections from Z-CAT fish injected with vehicle or 4-HT, at 7, 14 and 30 days post-injection (dpi). For each group, 5-7 animals were assessed. (B) Quantification of MHC+ myofiber area from experiments in A. (C) Quantification of Mef2+ cardiomyocyte nuclei from experiments in A. (D) Ventricular cardiomyocyte proliferation at 14 dpi assessed by Mef2 and PCNA staining. 4-HT-injected animals display widespread PCNA+ cardiomyocytes. (E) Quantification of ventricular cardiomyocyte proliferation in Z-CAT animals injected with vehicle or 4-HT, at 7, 14 and 30 dpi. For each group, seven to nine animals were assessed. *P<0.05, **P<0.005, Student′s t-test. Mean±s.e.m. Scale bars: 50 μm. |
|
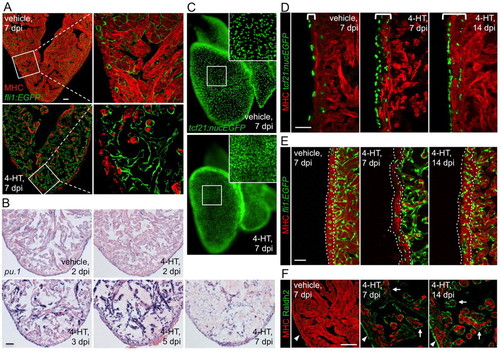
Effects of cardiomyocyte ablation on non-muscle cells. (A) Visualization of endocardial cells (green) and muscle (red) in sections from vehicle-or 4-HT-injected Z-CAT fish. The endocardial layer appears intact one week after massive cardiomyocyte loss. Single confocal slices are shown. (B) In situ hybridization for pu.1-expressing cells, which are prominent by 3-5 dpi. (C) Whole-mount visualization of epicardial nuclei in vehicle- or 4-HT-injected Z-CAT fish at 7 dpi, indicating higher epicardial cell density after myocardial injury. Inset shows enlarged view of boxed area. (D) Histological visualization of epicardial cells after myocardial ablation. Epicardial cells proliferate by 7 dpi and are incorporated into the regenerating myocardial wall by 14 dpi. Brackets indicate area containing tcf21-expressing epicardial cells. (E) Visualization of coronary vascular endothelial cells (green) in sections of the ventricular wall (outlined by dotted lines). Coronary vasculature is reduced in 4-HT-injected Z-CAT animals by 7 dpi, but restored by 14 dpi. (F) Sections from Z-CAT ventricles stained for the retinoic acid (RA)-synthesizing enzyme Raldh2. RA synthesis appears low in vehicle-injected tissue, but is strongly induced in epicardial (arrowheads) and endocardial (arrows) structures after cardiomyocyte ablation. Single confocal slices are shown. Scale bars: 50 μm. |
|
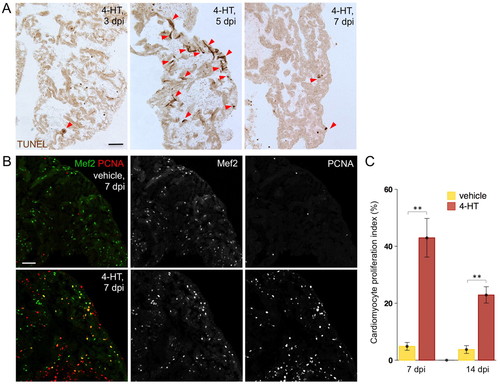
Atrial cardiomyocyte ablation and regeneration. (A) TUNEL staining of atrial sections from Z-CAT fish injected with 4-HT. Arrowheads indicate TUNEL-positive muscle. (B) Cardiomyocyte proliferation at 7 days post-injection (dpi) in atrial sections from Z-CAT animals injected with vehicle or 4-HT, assessed by Mef2 and PCNA staining. 4-HT-injected animals display widespread atrial PCNA+ cardiomyocytes. Scale bars: 50 μm. (C) Quantification of atrial cardiomyocyte proliferation in Z-CAT fish injected with vehicle or 4-HT, at 7 and 14 dpi. For each group, six to eight animals were assessed. Mean±s.e.m. **P<0.005, Student′s t-test. |

Lineage-tracing and ultrastructural analysis of regenerating muscle. (A) Schematic representation of transgenes used for myocyte ablation and lineage tracing. (B) Triple transgenic (Z-CAT; bactin2:loxp-DsRed-STOP-loxp-EGFP) zebrafish were injected once with vehicle (left) or 4-HT. In 4-HT-injected animals, EGFP labeled the majority of spared cardiomyocytes by 7 dpi (middle) and the majority of regenerated cardiomyocytes by 30 days post-injection (dpi; right). Arrowheads indicate examples of MHC+ tissue expressing EGFP. (C) Quantification of EGFP+ myocardium as a percentage of MHC+ ventricular tissue. At 7 dpi, 96% of myocardium was EGFP-labeled, and 95% at 30 dpi. For each group, 4-10 animals were assessed. Insets show enlarged views of the boxed area. (D) Assessment of sarcomere structure in Z-CAT fish crossed to a transgenic strain that labels Z-lines (green). Cardiomyocytes of control animals show well-organized sarcomeres with clear Z-lines. By contrast, many myocytes of 4-HT-injected animals display disorganized sarcomeres and loss of Z-lines (arrowheads) by 7 dpi and 14 dpi. By 30 dpi, sarcomeric structure and Z-lines are typically restored. Scale bars: 50 μm. (E) Transmission electron micrographs of ventricular myocytes from vehicle- or 4-HT-injected Z-CAT animals. Control myocytes have prominent sarcomeric structure and normal mitochondria (m), whereas 7 and 14 dpi myocytes appear less organized with fewer Z-lines (arrows) and swollen mitochondria (arrowheads). Scale bar: 0.5 μm. |
|
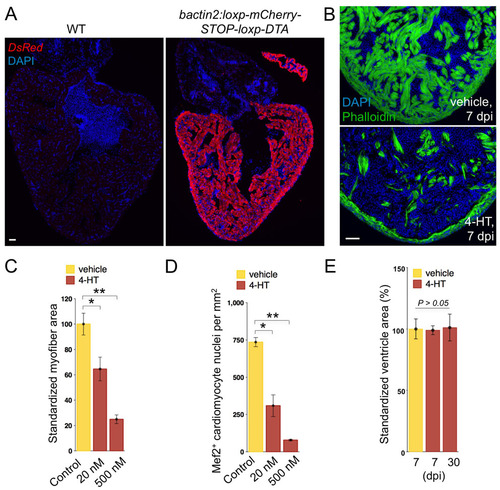
Robust, dose-dependent cardiomyocyte ablation. (A) Anti-DsRed staining to indicate mCherry expression in most if not all ventricular and atrial cardiomyocytes of transgenic animals (bactin2:loxp-mCherry-STOP-loxp-DTA). (B) Phalloidin staining indicates heavy cardiac myofiber loss at 7 days following a single 4-HT injection (bottom), compared with vehicle control (top). (C,D) Quantification of myofiber area by MHC (C) or Mef2 (D) staining in Z-CAT fish incubated with vehicle (n=5) or two different concentrations of 4-HT (20 nM, n=5; 500 nM, n=3) for 12 hours. Ablation was assessed at 7 days after incubation in 4-HT. Mean±s.e.m. **P<0.005, *P<0.05, Studentýs t-test. (E) Ventricular section surface area was measured using Openlab software, using the three largest sections from each ventricle. Five or six animals were assessed for each group. Scale bars: 50 μm. |
|
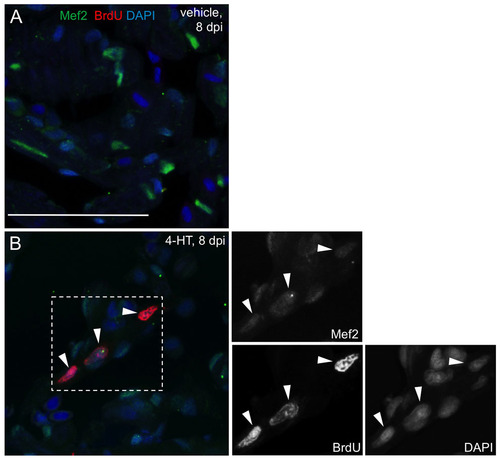
BrdU incorporation after cardiomyocyte ablation. (A,B) BrdU-labeled Mef2-positive nuclei in ventricles from Z-CAT animals treated with vehicle (A) or 4-HT (B) by 8 dpi, one day after a single injection of BrdU. Single confocal slices are shown. Arrowheads indicate nuclei positive for both BrdU and Mef2. Panels on the right show enlarged view of boxed area in B. Scale bar: 50 μm. |
|
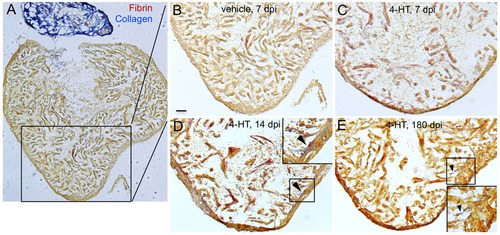
Scarring is not a component of ablation-induced cardiac regeneration. (A-E) Ventricular sections from Z-CAT fish injected with vehicle (A,B) or 4-HT (C-E) were stained with Acid Fuchsin-Orange G (AFOG; fibrin, orange/red; collagen, blue). 4-HT-injected animals occasionally displayed minor collagen deposition, evident in the 14 dpi and 180 dpi samples (arrowheads). Scale bar: 50 μm. |
|
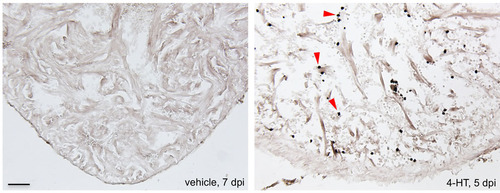
Neutrophil recruitment after cardiomyocyte ablation. Myeloperoxidase reactivity (black) in sections from vehicle-or 4-HT-injected Z-CAT fish, indicative of neutrophils (arrowheads). Scale bar: 50 μm. |