- Title
-
Pou5f1 contributes to dorsoventral patterning by positive regulation of vox and modulation of fgf8a expression
- Authors
- Belting, H.G., Wendik, B., Lunde, K., Leichsenring, M., Mössner, R., Driever, W., and Onichtchouk, D.
- Source
- Full text @ Dev. Biol.
|
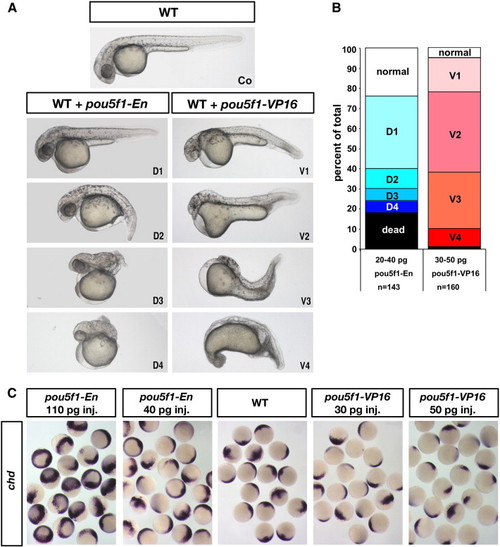
Overexpression of Pou5f1-VP16 can ventralize wild-type embryos while overexpression of Pou5f1-En can dorsalize. (A) Live photographs of a wild-type (WT) zebrafish embryo at 33–36 h post fertilization (28.5 °C) (top) and of embryos that were injected with pou5f1-En mRNA (20 pg; D1–D4) or pou5f1-VP16 mRNA (40 pg; V1–V4) at the one-cell stage within 30 min of egg laying. As an injection control, membrane-GFP mRNA (30 pg) was co-injected, and embryos with low GFP expression at early shield stage were removed. Lateral views, anterior to left, dorsal at top. Dorsoventral index modified from Kishimoto et al. (1997). (B) Dorsoventral index of WT zebrafish embryos scored at 33–36 hpf. The graph shows percent of phenotypes (V1–V4, D1–D4 as in (A), dead and normal) from total number of injected embryos. (C) Whole-mount in situ hybridization of pooled WT embryos injected with pou5f1-VP16 mRNA (30 or 50 pg) or pou5f1-En mRNA (40 or 110 pg) at the one-cell stage, and of non-injected WT control embryos, showing expression of chordin at 70% epiboly. |
|
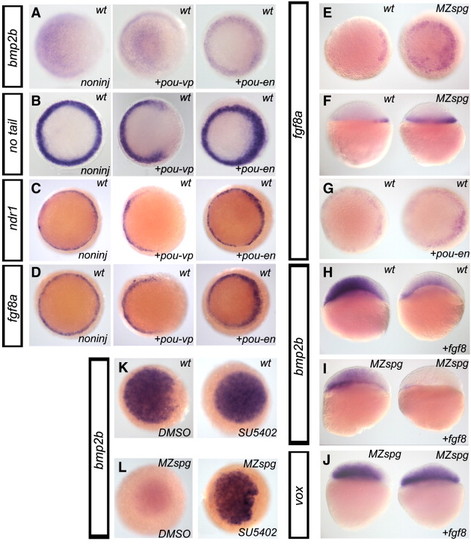
Regulatory interactions of pou5f1, fgf8a and bmp2b. Whole-mount in situ hybridization of wild-type (WT) embryos and MZspg mutants, injected with mRNAs as indicated. (A–D) WT embryos were left non-injected, or were injected with Pou5f1-VP16 (pou-vp) or Pou5f1-Engrailed (pou-en) encoding mRNAs, fixed at 40%–50% epiboly stage, and stained with the probes as indicated on the left sides of the panels. Animal views. Note the gap in the marginal ring of expression of no tail, ndr1 and fgf8a for Pou5f1-VP16 overexpressing embryos, and local thickening of the marginal expression ring for Pou5f1-Engrailed overexpressing embryos (B–D). (E, F) Sphere stage WT embryos and MZspg mutants probed with fgf8a. fgf8a is expanded in MZspg embryos. (C) Animal view, (D) lateral view. (G) WT embryos were left non-injected (left), or were injected pou5f1-En mRNA (43 pg; right) and probed with fgf8a. fgf8ais expanded in pou5f1-En mRNA injected embryos (7, n = 8). Animal view, sphere stage. (H) WT embryos were left non-injected (left), or were injected with fgf8a mRNA (3 pg/embryo; right) and probed with bmp2b. bmp2b expression is reduced in 12 out of 14 fgf8a mRNA injected WT embryos analyzed. Lateral view, 30% epiboly. (I, J) FGF signaling is functional in blastula MZspg embryos and can suppress bmp2b expression. MZspg embryos were left non-injected (left), or were injected with fgf8a mRNA (3 pg/embryo) and probed with bmp2b (G) or vox (H). (G) bmp2b expression is further reduced in fgf8a mRNA injected MZspg embryos (13, n = 14 embryos). (H) vox expression did not significantly change. Lateral views 30% epiboly. (K, L) Inhibition of FGF signaling restores bmp2b expression at sphere stage in MZspg embryos. To inhibit FGF signaling, WT (K) and MZspg (L) embryos were treated with 40 µM SU5402 (or controls with DMSO only) starting from 2-cell stage, fixed at sphere stage, and probed with bmp2b. |
|
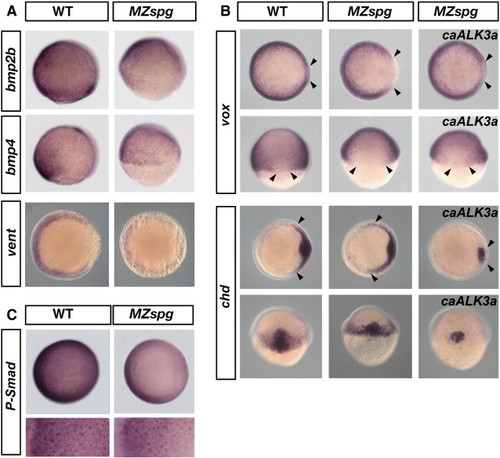
BMP signaling and expression of zygotic BMP pathway genes in MZspg. (A) Expression of BMP pathway genes is reduced in MZspg embryos. From top to bottom: Whole-mount in-situ hybridization of wild-type (WT) embryos and MZspg mutants showing expression of bmp2b, bmp4 (lateral view, dorsal to the right, 75% epiboly) and vent (animal view, dorsal to the right, shield stage). (B) Constitutively active BMP receptor 1a (caALK3a) can rescue dorsalization of MZspg embryos at midgastrulation. Whole-mount in situ hybridization of wild-type (WT) embryos, MZspg mutants, and MZspg mutants injected with caALK3a mRNA, as indicated. From top to bottom: vox at 70% epiboly, chordin at shield stage; top rows: animal pole views, dorsal at right; bottom row: dorsal view, animal pole up. Arrows show the dorsolateral borders of vox and chd staining. caBMPR1a mRNA (100–150 pg) was injected into wild-type (WT) embryos at the one-cell stage. (C) Immunostaining for Phospho-Smad 1/5/8 (animal view, dorsal at right) of WT and MZspg embryos at 75% epiboly. Lower panel shows animal-ventral view of the embryos in higher magnification, to visualize P-Smad nuclear staining from ventral margin (left) toward the animal pole (right). |
|
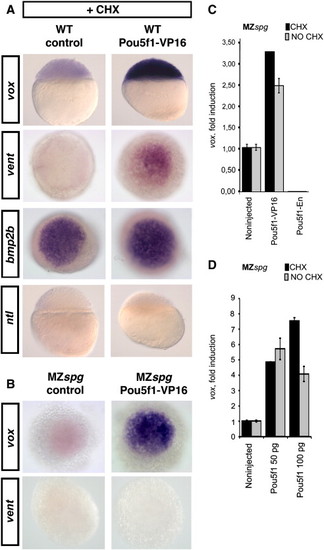
vox is a direct transcriptional target of Pou5f1. (A) Whole-mount in situ hybridization of 5 hpf wild-type control embryos and embryos injected with Pou5f1-VP16; probes are as indicated. Embryos were treated with cycloheximide (CHX) starting at the 64-cell stage to prevent translation. Absence of ntl staining served as a control for zygotic translation block. Note that Pou5f1-VP16 induced vent and vox genes. Embryos probed with ntl and vox are shown in lateral view, and embryos probed with bmp2b and vent stained embryos are shown in animal view. (B) Whole-mount in situ hybridization of CHX-treated non-injected MZspg embryos and MZspg embryos injected with pou5f1-VP16 and analyzed with vox or vent probes, animal view (44 out of 48 embryos showed increased vent expression). (C, D) Quantitative PCR: vox mRNA levels were measured in CHX treated and untreated shield stage MZspg embryos, which were non-injected, or injected with mRNAs at one cell stage as indicated. vox expression values were normalized to housekeeping gene ef1alpha expression levels (ddCT method) and then to non-injected control (set as 1). (C) Pou5f1-VP16 induces vox, and Pou5f1-En repressed vox expression in the presence of CHX in MZspg. (D) Pou5f1 induces vox in the presence of CHX in MZspg. |
Reprinted from Developmental Biology, 356(2), Belting, H.G., Wendik, B., Lunde, K., Leichsenring, M., Mössner, R., Driever, W., and Onichtchouk, D., Pou5f1 contributes to dorsoventral patterning by positive regulation of vox and modulation of fgf8a expression, 323-36, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.