- Title
-
Compartmentalized Notch signaling sustains epithelial mirror symmetry
- Authors
- Wibowo, I., Pinto-Teixeira, F., Satou, C., Higashijima, S., and Lopez-Schier, H.
- Source
- Full text @ Development
|
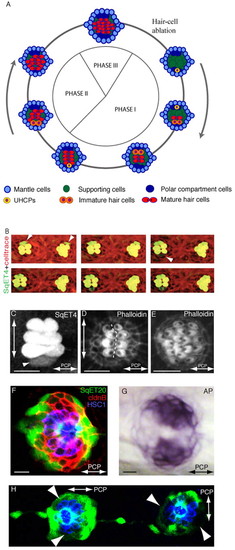
Epithelial planar polarization of neuromasts. (A) Schematic description of the three phases of regeneration and homeostasis and the planar polarization of the neuromast epithelium (macula). Cell types are color-coded: most peripheral mantle cells (light blue), central supporting (sustentacular) cells (green), polar compartment resident cells (dark blue), UHCPs (yellow), immature hair cells (orange) and mature hair cells (red). The axis of planar polarity is evidenced by the position of the kinocilium within the mature hair cells (black dot). (B) Time-lapse of two adjacent parallel SqET4 neuromasts showing hair cells and their progenitors (green) and stained with Celltrace to reveal cellular boundaries (red). White arrowheads on the first panel indicate a pair of newly developed hair cells on the dorsum of both neuromasts. The white arrowhead on the third panel indicates a new ventral UHCP, which divides into two hair cells on the last panel. Hair cells develop in pairs, sequentially, and on the dorsal or ventral aspect of the neuromasts. (C) A regenerating SqET4 neuromast revealing six GFP-positive hair cells. A UHCP develops at the lower edge of the neuromast (arrowhead). The orthogonal axes of planar polarization (horizontal) and direction of regeneration (vertical) are indicated by double-headed arrows. (D) Actin staining of a regenerating neuromast, showing the orientation of four pairs of hair bundles and revealing the vertical line of bilateral symmetry (dotted white line). (E) Actin staining of neuromast in Phase III of regeneration showing the lateral expansion of the macula. A double-headed arrow indicates the axis of planar polarity. (F) A SqET20 neuromast expressing GFP (green) and stained with antibodies to Claudin-b (red), which marks all the supporting cells, and to HCS1 (blue), which highlights the hair cells. GFPlow compartments are located at the poles of the vertical axis of bilateral symmetry. (G) The GFPlow compartments coincide with the endogenous expression of alkaline phosphatase. In all figures, anterior is leftwards and dorsal is upwards. (H) A SqET20 larva expressing GFP (green) and stained with the antibody HCS1 (blue). The neuromast to the left of the image is L1 (a parallel neuromast), whereas the one on the right is L2 (a perpendicular neuromast). This image shows that the GFPlow compartments rotate through 90° in parallel versus perpendicular neuromasts. Arrowheads indicate polar compartments. Scale bars: 10 μm. |
|
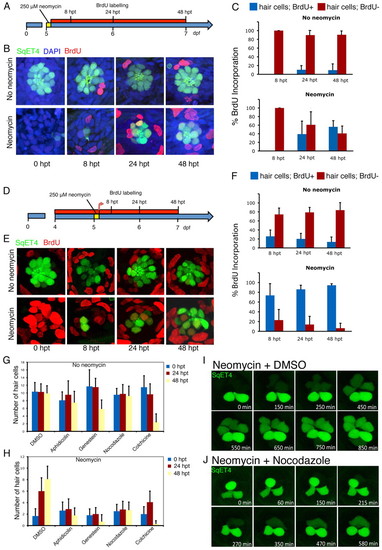
Mitotic activity is essential for hair-cell regeneration neuromasts. (A) Scheme representing the experiments in which hair cells were ablated with neomycin in SqET4 transgenic zebrafish larvae at 5 dpf, and subsequently incubated continuously in BrdU for 8, 24 and 48 hours after neomycin treatment (8,24 and 48 hpt). (B) Confocal images of neuromasts showing hair cells in green and BrdU in red. All cell nuclei were labeled with DAPI (blue). (C) The mean number of GFP(+) hair cells that were BrdU(+) versus BrdU(-) in control and treated larvae at 8, 24 and 48 hpt (n=70 neuromasts for each timepoint). In control samples not treated with neomycin, few hair cells were labeled with BrdU at 48 hpt, whereas 59% of regenerated hair cells were BrdU(+) in neomycin-treated fish at 48 hpt. Error bars indicate s.e.m. (D) The experiments in which SqET4 transgenic zebrafish at 4 dpf were incubated continuously in BrdU, treated with neomycin to ablate hair cells at 5 dpf and subsequently incubated continuously in BrdU for 8, 24 and 48 hpt (results are shown in E,F). (E) Confocal images of neuromasts showing hair cells in green and BrdU in red. (F) In control samples not treated with neomycin, 74% of regenerated hair cells at 8 hpt are BrdU positive (n=70), compared with only 26% BrdU-positive hair cells in untreated larvae (n=70). By 48 hpt, 94% of regenerated hair cells are BrdU(+), compared with only 13% in untreated specimens. This finding indicates that virtually all regenerated hair cells develop from progenitors that have undergone cell division following neomycin-induced hair-cell ablation and that progenitors enter the cell cycle less than 8 hours after hair-cell death. Results are mean;;plusmn;s.e.m. (G,H) SqET4 transgenic larvae were incubated in several inhibitors of the cell cycle for 24 and 48 hours following neomycin treatment. The quantification shows that the number of hair cells remains relatively constant following treatments with the cell-cycle blockers aphidicolin, genistein and nocodazole in control samples (not treated with neomycin). All cell-cycle inhibitors blocked hair-cell regeneration in samples treated with neomycin. Results are mean±s.e.m. (I,J) These results were confirmed by time-lapse videomicroscopy in regenerating SqET4 control (I) and nocodazole-treated (J) larvae. Controls regenerated normally, whereas nocodazole blocked regeneration and the division of a UHCP that appears at the top of the neuromast in J. |
|
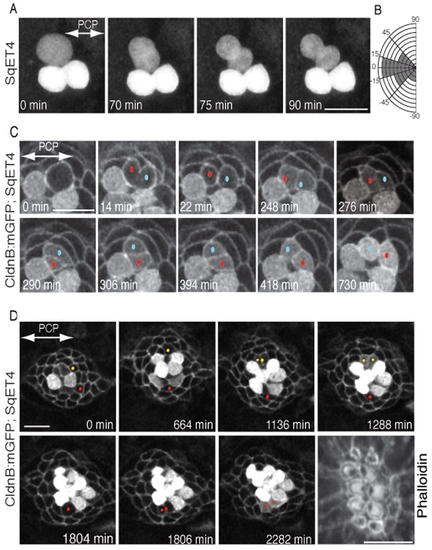
Orientation of UHCP divisions and planar cell inversion. (A) A 90-minute series of confocal images of a regenerating SqET4 neuromast revealing two GFP-positive hair cells aligned along the axis of planar polarity (double-headed arrow). An UHCP develops at the upper edge of the neuromast. Over the next hour, this UHCP commences mitosis and divides obliquely into a pair of hair cells. (B) Quantification of the angle of division of UHCPs in 23 neuromasts, which indicates that the majority of UHCP divide obliquely respect to the axis of planar polarity of the epithelium. (C) A 12-hour series of confocal images of a regenerating neuromast of a double-transgenic Tg[Cldnb:mGFP;SqET4] larva revealing two GFP-positive hair cells at the lower aspect of the image, and a UHCP at the top. At 14 minutes into the time-series, this UHCP divides into two hair cells, each identified with a dot (red and blue). At 264 minutes after cytokinesis (2762), the sibling hair cells begin to rotate around their contact point within the plane of the epithelium, which locally breaks the line of mirror symmetry (this is most evident at 306 minutes into the time series). A complete inversion of the hair-cell pair eventually reforms the line of mirror symmetry and precise realigns the cells along the axis of planar polarity of the neuromast (double-headed arrow in the first panel). (D) A 44-hour series of confocal images of a regenerating neuromast of a double-transgenic Tg[Cldnb:mGFP;SqET4] larva reveals plasma-membrane GFP-positive supporting cells and cytoplasmatic GFP-positive hair cells and UHCPs. Two prospective UHCPs were identified retrospectively by playing the time series backwards, and each labeled with either a red or yellow dot. Their position was followed over time, showing that prospective UHCPs moved into the dorsal (yellow) and ventral (red) polar compartments, where they became UHCPs (weak cytoplasmatic GPF). Each UHCP eventually divided into a pair of hair cells. The last panel of the image is an actin staining, showing the resulting planar polarization of the epithelium. The axis of planar polarity of the neuromast is indicated by a double-headed arrow in the first panel. Scale bars: 10 μm. |
|
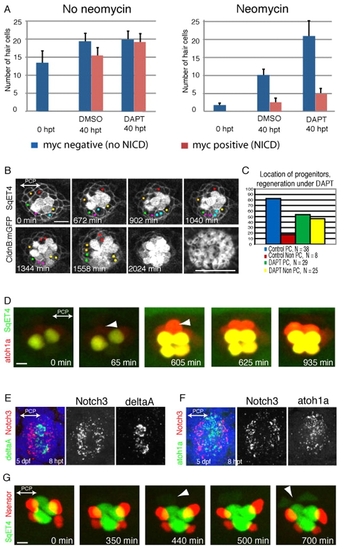
UHCP develop in polar compartments. (A) Quantification of the average number of hair cells in samples that were treated with neomycin (right) or that were not treated (left), and later exposed to DMSO or DAPT, and that expressed (red) or did not express (blue) a constitutively active form of Notch. It shows that DAPT induces the overproduction of hair cells, and this effect is suppressed by Notch activity. Results are mean±s.e.m. (B) A 34-hour time series of a regenerating Tg[Cldnb:mGFP; SqET4] neuromast treated with DAPT. UHCPs were identified retrospectively by playing the time series backwards, and labeled with colored dots. UHCPs develop in excess, producing pairs of hair cells ectopically. Actin staining shows the resulting defective planar polarization (last panel). (C) A graph showing the position of UHCPs relative to the polar compartments in control and DAPT-treated neuromasts during regeneration. More than 80% of UHCPs develop within the polar compartments in controls, compared with just over 50% in DAPT-treated samples (n=25). Results are mean±s.e.m. (D) Time series of a Tg[Atoh1a:TdTomato; SqET4] double-transgenic neuromast revealing GFP-positive UHCPs and hair cells (green), and Atoh1a-expressing cells (red). It shows the temporal gene-expression hierarchy. Arrowheads indicate UHCPs. (E,F) Fluorescent whole-mount in situ hybridizations of regenerating neuromasts, revealing Notch3 (red) and DeltaA (green) (E), and Notch3 (red) and Atoh1a (green) (F). Cell nuclei are in blue. Notch3 is never expressed by the DeltaA(+) or Atoh1a(+) cells, and was absent from the polar compartments. (G) A 700-minute live imaging of a double-transgenic SqET4 (green) neuromast expressing a red-fluorescent Notch sensor (red). Notch signaling occurs outside the polar compartments. Arrowheads indicate UHCPs. |
|
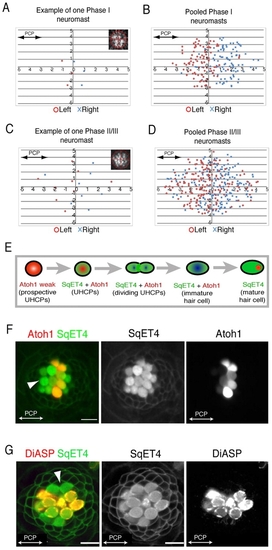
Local and global planar polarization of the neuromast epithelium. (A-D) Hair-cell planar polarity distribution in regenerating and mature neuromasts. Positional values of each polarity are plotted in a Cartesian plane. (A,B) Example of one neuromast (A) and 20 pooled neuromasts (B) in Phase I. (C,D) Example of one neuromast (C) and 22 pooled neuromasts (D) in Phase II/III. (E) Schematic transition of marker gene-expression in prospective UHCPs [weakly Atoh1a+ (pink)], UHCPs, immature and young hair cells [co-expressing SqET4 and Atoh1a (green/red)], and mature hair cells [exclusively SqET4+ (green)]. (F) A Tg[Atoh1a:TdTomato; SqET4] neuromast exemplifies this transition [GFP(+) UHCPs and hair cells (green) and Atoh1a(+) cells (red)]. The white arrowhead indicates mature hair cells on the rostral part of this parallel neuromast. (G) DiASP incorporation into hair cells of Tg[Cldnb:mGFP; SqET4] neuromast, showing the exclusion of mature hair cells from the polar compartments. The white arrowhead indicates immature hair cells on the dorsal part of this parallel neuromast. |