- Title
-
Cilia-driven fluid flow as an epigenetic cue for otolith biomineralization on sensory hair cells of the inner ear
- Authors
- Yu, X., Lau, D., Ng, C.P., and Roy, S.
- Source
- Full text @ Development
|
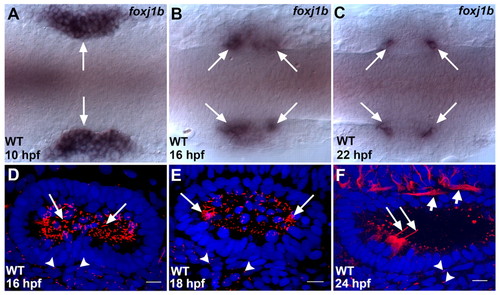
Expression of foxj1b and the distribution of cilia in the zebrafish otic vesicle. (A) In the wild type (WT), foxj1b is initially expressed throughout the otic placodes (arrows). (B) Restriction of foxj1b expression to the two poles of the otic placodes (arrows) at 16 hpf. (C) foxj1b expression finally gets restricted to a small cluster of cells at the anterior and posterior ends of the otic vesicles (arrows). (D) Large numbers of cilia (arrows) initially populate the otic vesicle. (E) Subsequently, most of the cilia disappear, except at the two poles (arrows). (F) At 24 hpf, hair cell kinocilia are clearly visible at the two poles (long arrows), whereas cilia in the rest of the vesicle have regressed. The ears of embryos shown in A-C are labeled with foxj1b antisense RNA probe, whereas those in D-F are labeled with anti-acetylated tubulin antibodies (ciliary axonemes, red) and with DAPI (nuclei, blue). In D-F, primary cilia in cells adjoining the otic vesicles are indicated (arrowheads). In F, the short arrows mark neuronal processes which are also labeled by the anti-acetylated tubulin antibodies. A-C, dorsal views; D-F, lateral views of otic vesicles. Scale bars: 10 μm. |
|
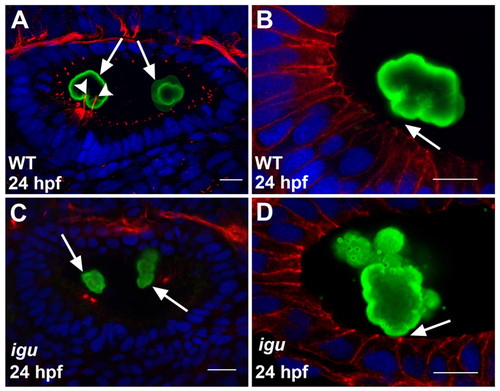
Motile cilia are crucial for proper otolith formation. (A) Wild-type zebrafish ear showing the deposition of the two otoliths (green, arrows) at the poles of the otic vesicle. Note the two kinocilia (red, arrowheads) bearing the anterior otolith. (B) High-resolution image of a wild-type ear showing the anterior otolith (green). Note the gap (arrow) between the otolith surface and the apical membranes of the hair cells (red). (C) Ear of an igu mutant embryo, showing irregularly shaped otoliths (green) at the two poles (arrows) and the complete absence of cilia. (D) High-resolution image of an ear of an igu mutant showing an irregular otolith (green) attached to the apical membranes of the hair cells (red, arrow). Otoliths of embryos shown in A-D were stained with antibodies to Starmaker (Stm) (green), a component of the otoliths (Sollner et al., 2003), cilia in A and C with anti-acetylated tubulin antibodies (red), and cell membranes in B and D with anti-β-catenin antibodies (red). Nuclei were visualized with DAPI (blue). All panels show lateral views of otic vesicles with anterior to the left. Scale bars: 10 μm. |
|
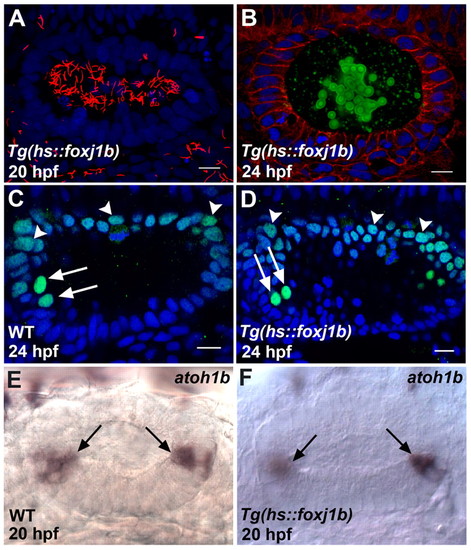
Proper otolith formation requires a specific spatiotemporal distribution of motile cilia. (A) Ear of a zebrafish embryo overexpressing foxj1b, with large numbers of long motile cilia (red) throughout the otic vesicle. (B) Ear of a foxj1b-overexpressing embryo showing defective otolith (green) formation. (C) Wild-type pattern of Pax2 expression (green) in the dorsal part of the otic vesicle (arrowheads) and in the hair cells (arrows). The two anterior hair cell nuclei (arrows) are identifiable by the relatively high levels of Pax2 expression. (D) Pax2 expression (green) is unaltered in the dorsal part of the otic vesicle (arrowheads) and in the hair cells (arrows) of a foxj1b-overexpressing embryo. The two anterior hair cell nuclei are indicated (arrows). (E) Wild-type pattern of atoh1b expression in the otic vesicle. The hair cells at the two poles are indicated (arrows). (F) atoh1b expression is unaffected in the ear of a foxj1b-overexpressing embryo. The hair cells at the two poles are indicated (arrows). Cilia of the embryo in A were stained with anti-acetylated tubulin antibodies (red), cell membranes of the embryo in B with anti-β-catenin antibodies (red) and otolith particles with anti-Stm antibodies (green), and the embryos in C and D with anti-Pax2 antibodies (green). Nuclei in A-D were visualized with DAPI (blue). All panels show lateral views of otic vesicles with anterior oriented to the left. Scale bars: 10 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
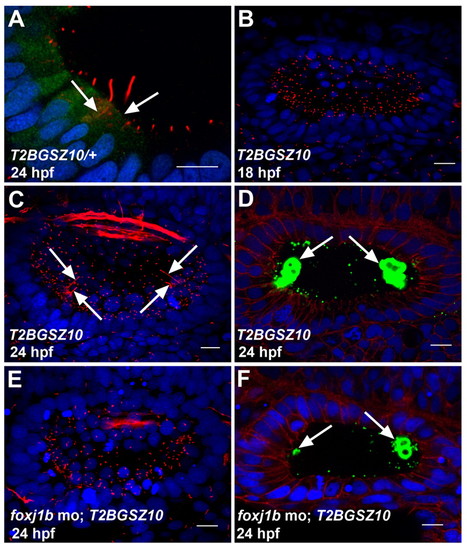
Foxj1b is required for the differentiation of motile and kinocilia. (A) Expression of GFP in the hair cells of a heterozygous T2BGSZ10 transgenic zebrafish embryo. Arrows indicate hair cells. (B) Defective motile cilia differentiation in the ear of a homozygous T2BGSZ10 transgenic embryo (compare with Fig. 1E). (C) Defective kinocilia differentiation in the ear of a homozygous T2BGSZ10 transgenic embryo. Note the variable lengths of the kinocilia (arrows) (compare with Fig. 1F). (D) Malformed otoliths (green, arrows) in the ear of a homozygous T2BGSZ10 transgenic embryo. (E) A foxj1b morpholino-injected homozygous T2BGSZ10 transgenic embryo showing a more pronounced effect on ciliary differentiation with severe reduction in the motile and kinocilia. (F) A more prominent effect on otolith (green, arrows) formation in the ear of a foxj1b morpholino-injected homozygous T2BGSZ10 transgenic embryo. Embryos in A-C,E were stained with anti-acetylated tubulin antibodies (red), and those in D and F with anti-Stm antibodies (green). The embryo in A was co-stained with anti-GFP antibodies (green), whereas those in D and F were co-stained with anti-β-catenin antibodies (red). Nuclei were visualized with DAPI (blue). All panels show lateral views of otic vesicles with anterior to the left. Scale bars: 10 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
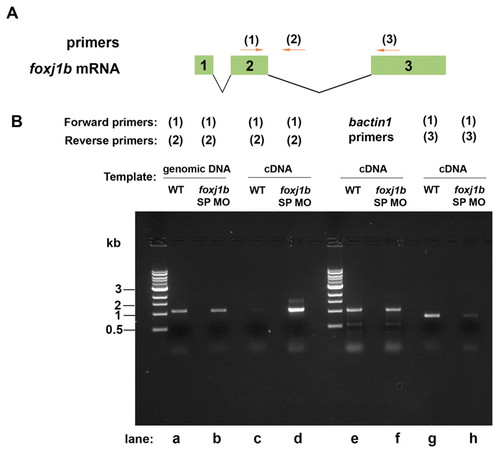
Effect of the foxj1b splice-inhibitory morpholino on splicing of foxj1b mRNA. (A) The positions of foxj1b primers (1-3) are shown relative to the exon/intron positions of zebrafish foxj1b mRNA. (B) RT-PCR with foxj1b primers (1) and (2) showing an increase in the amount of unspliced foxj1b transcript in the foxj1b splice morpholino (SP MO)-injected embryos (lanes c and d). RT-PCR with foxj1b primers (1) and (3) showed a decrease in the level of foxj1b transcript in the foxj1b SP MO-injected embryos (lanes g and h). bactin1 mRNA levels were unaffected (lanes e and f). |
|
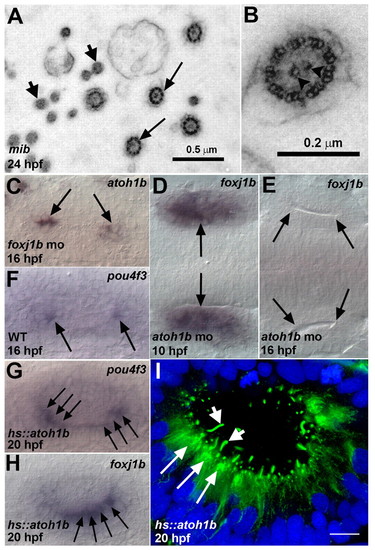
Atoh1b-dependent regulation of foxj1b in kinocilia differentiation. (A) Electron micrograph (transverse section) of kinocilia (arrows) from the ear of a mib mutant zebrafish embryo. The microvilli (stereocilia) of the hair cells are also visible (small arrows). (B) High-resolution image showing the 9+2 arrangement of microtubules. The central pair of microtubules is indicated (arrowheads). (C) Wild-type pattern of atoh1b expression in the otic vesicle of a foxj1b morphant (compare with Fig. 3E). (D) Normal pattern of foxj1b expression in the otic placode of an atoh1b morphant at 10 hpf (compare with Fig. 1A). (E) Loss of foxj1b expression (arrows) from the ear of an atoh1b morphant at 16 hpf (compare with Fig. 1B). (F) Expression of pou4f3 in the differentiating hair cells (arrows) of a wild-type ear. (G) Ectopic pou4f3 expression (arrows) in supernumerary hair cells in the ear of a Tg(hs::atoh1b) transgenic embryo. (H) Ectopic foxj1b expression (arrows) in supernumerary hair cells in the ear of a Tg(hs::atoh1b) transgenic embryo (compare with Fig. 1C). (I) Kinocilia (small arrows) on ectopic hair cells (long arrows) in the ear of a Tg(hs::atoh1b) transgenic embryo. The embryo was stained with antibodies to acetylated tubulin (green) and with DAPI (blue). Scale bar: 10 μm in I. EXPRESSION / LABELING:
|