- Title
-
Zebrafish Tie-2 shares a redundant role with Tie-1 in heart development and regulates vessel integrity
- Authors
- Gjini, E., Hekking, L.H., Küchler, A., Saharinen, P., Wienholds, E., Post, J.A., Alitalo, K., and Schulte-Merker, S.
- Source
- Full text @ Dis. Model. Mech.
|
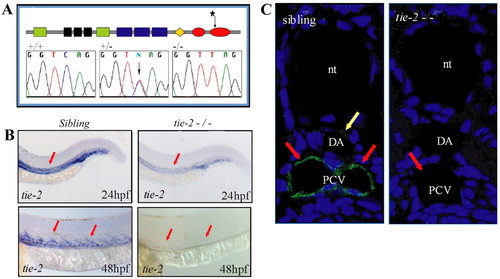
A mutation in the kinase domain of tie-2 leads to loss of Tie-2 protein. (A) Schematic of the Tie-2 protein domains. Green, black and blue boxes represent the Ig-like, EGF-like and FN-III protein domains, respectively. The orange diamond represents the transmembrane region and the two red ovals represent the two kinase domains. Sequence chromatograms show the tie-2hu1667 allele to encode a stop codon within the second kinase domain. Sequence of the tie-2 hu1667 allele: CAG (Q982) is changed into a TAG (STOP) codon (mutation indicated by a star). (B) At 24 hpf, tie-2 mRNA expression levels are significantly reduced in tie-2 mutants compared with wild-type siblings (red arrows). tie-2 mRNA expression is absent at 48 hpf in mutants, whereas it is readily detectable in wild-type siblings (red arrows). (C) In wild-type fish, Tie-2 protein is expressed in the PCV at 48 hpf. Tie-2 protein expression is completely undetectable in tie-2 mutants. Yellow arrow shows Tie-2 protein expression in the dorsal aorta (DA); red arrows show Tie-2 protein expression in the PCV. nt, notochord. |
|
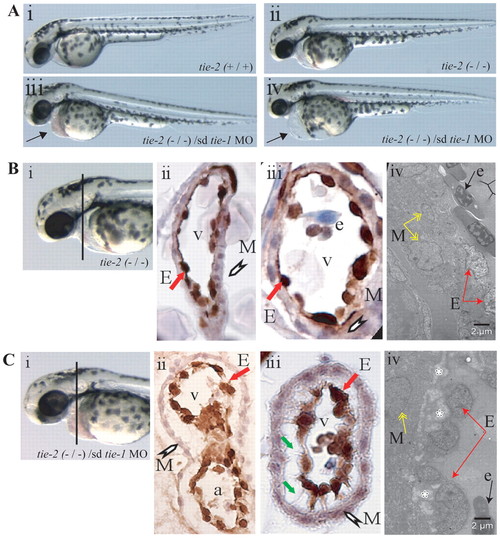
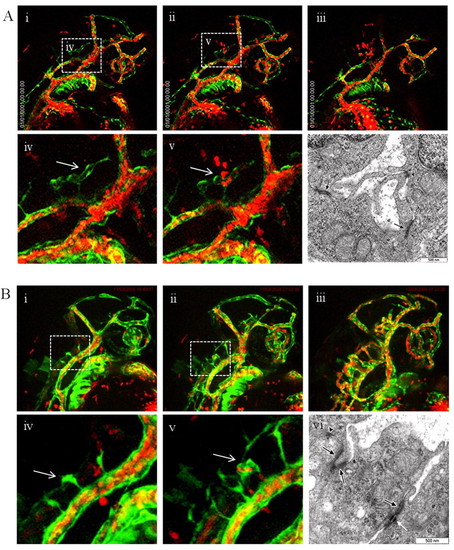
Loss of tie-1 function in tie-2 mutants results in severe defects in endocardial-myocardial contact. (A) Wild type (i) and tie-2 mutants (ii) show no cardiac defects, whereas tie-1 morpholino knockdown in tie-2 mutants (iii,iv) leads to blood accumulation (arrow in iii) in the pericardial sac or to cardiac edema (arrow in iv) and arrested blood circulation (iv). (B) Immunohistochemistry using an anti-GFP antibody on heart cross-sections from 3-dpf tie-2 mutants in tg(fli1:EGFP)y1 background (i–iii) reveals no defects in myocardial (white arrowhead) and endocardial (red arrow) contact. (Biv) See below. (C) Immunohistochemistry using an anti-GFP antibody on heart cross-sections from 3-dpf tie-2 mutants in the tg(fli1:EGFP)y1 background injected with sd-tie-1 MO (i–iii) reveals defects in myocardial (white arrowhead) and endocardial (red arrow) contacts. Endothelial cells exhibit thin cytoplasmic protrusions (green arrows) attached to the myocardium. (Biv,Civ) EM studies demonstrate defects (depicted by white asterisks) in endocardial (red arrows) and myocardial (yellow arrows) lining in tie-2 mutants injected with sd-tie-1 MO (Civ) but not in control tie-2 mutants (Biv). Black arrow depicts an erythrocyte. M, myocardium; E, endocardium; v, ventricle; e, erythrocyte; a, atrium. EXPRESSION / LABELING:
PHENOTYPE:
|
|
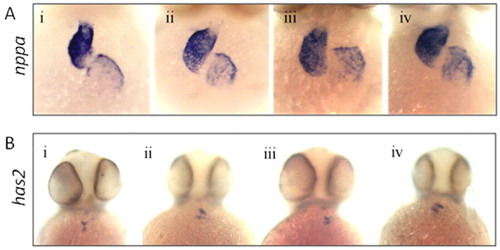
Loss of Tie-1 and Tie-2 signaling does not affect nppa and has2 expression. Whole-mount in situ hybridization for nppa (A) and has2 (B) in wild-type embryos (Ai; Bi), tie-2 mutants (Aii; Bii), wild-type embryos injected with sd-tie-1 MO (Aiii; Biii) and tie-2 mutants injected with sd-tie-1 MO (Aiv; Biv). Embryos were fixed at 48–50 hpf; all pictures were taken as ventral view. |
|
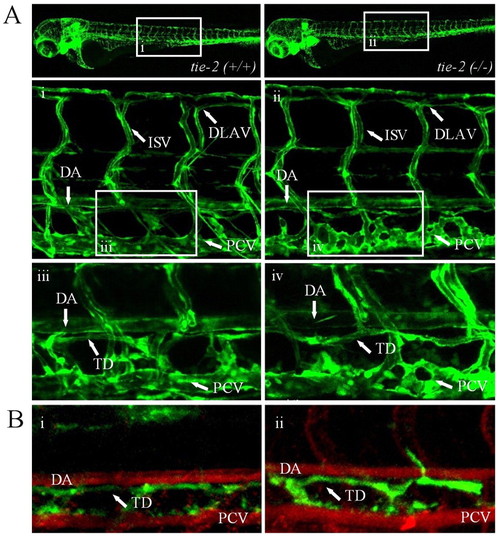
tie-2 mutants develop normal blood and lymphatic vasculature. (A) Confocal microscopy of trunk vessels in 5-day-old wild-type (Ai) and tie-2 mutant (Aii) embryos in the Tg(fli1:EGFP) background. Loss of Tie-2 protein does not affect blood and lymphatic vessel formation (Aii,iv) in zebrafish as compared to the wild-type blood (Ai) and lymphatic (Aiii) vessel development. (B) A test for functionality of the thoracic duct shows dye uptake in both wild-type siblings (Bi) and tie-2 mutants (Bii). Angiographies were performed in Bi and Bii to highlight the dorsal aorta (DA) and PCV. ISV, intersegmental vessels; TD, thoracic duct; DLAV, dorsal longitudinal anastomotic vessel. |
|
Lack of Tie-2 protects zebrafish embryos from cranial hemorrhages caused by treatment with statins. (A) Graphic representation (in percentage) of hemorrhagic (orange bars) and non-hemorrhagic (yellow bars) groups for wild-type siblings (i) and tie-2 mutants (ii) after exposure to increasing concentrations of atorvastatin; the numbers of hemorrhagic (orange bars) and non-hemorrhagic (yellow bars) groups for wild-type siblings (iii) and tie-2 mutants (iv) after exposure to increasing concentrations of simvastatin are shown. (B) tie-2 mutants show similar cranial hemorrhages (arrow) as the wild-type embryos after treatment with atorvastatin. (C) No cranial hemorrhages are observed in wild-type siblings and tie-2 mutants as shown by o-dianizidine staining. However, treatment with 1000 nM atorvastatin causes cranial hemorrhages in the head region of both genotypic groups (asterisks show the regions in the head area of siblings and mutants where blood is accumulating). PHENOTYPE:
|
|
Atorvastatin causes bleeding through vessel rupture. (A) Time-lapse imaging shows that atorvastatin causes vessel rupture and thus bleedings in siblings at the region of the central arteries (i–v). Ruptures occur precisely at the moment when blood enters the lumenized vessel (white arrow in iv and v). (vi) EM analysis in the head region of non-exposed siblings shows no defects in endothelial cell-cell contact. Black arrows indicate the junctional complex. (B) Time-lapse imaging shows that atorvastatin does not cause vessel rupture and thus bleedings in the tie-2 mutants at the region of the central arteries (i–v). No rupture occurs when blood enters the lumenized vessel (white arrow in iv and v). (vi) EM analysis in the head region of non-exposed tie-2 mutants shows no defects in endothelial cell-cell contact. Black arrows indicate the junctional complexes; white arrow indicates a junctional structure with desmosomal components; arrowhead indicates a junctional structure. Scale bars: 500 nm. |
|
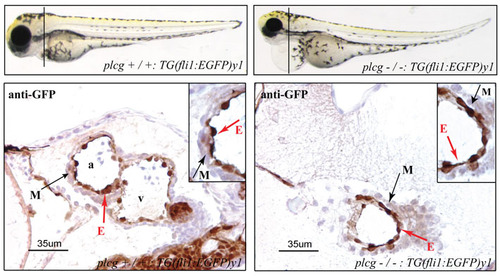
plcg mutants show no defects in endocardial-myocardial interaction. Anti-GFP antibody staining on heart sections of plcg mutant at 3.5 dpf reveal no defects in endocardial-myocardial connection. |
|
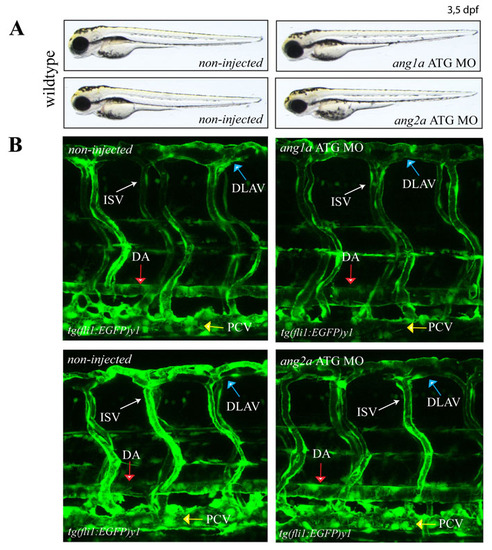
Knockdown of ang1a and ang2a leads to normal vasculature development in zebrafish. Knockdown of either the ang1a and ang2a gene in the zebrafish does not affect normal development of zebrafish embryos (A) and blood vasculature in the trunk as shown in the tg(fli1:EGFP)y1 background (B). DA (Dorsal Aorta; red arrow), PCV (Posterior Cardinal Vein; yellow arrow), DLAV (Dorsal Longitudinal Anastomotic Vessel; blue arrow), ISV (Intersegmental Vessels; white arrow) |
|
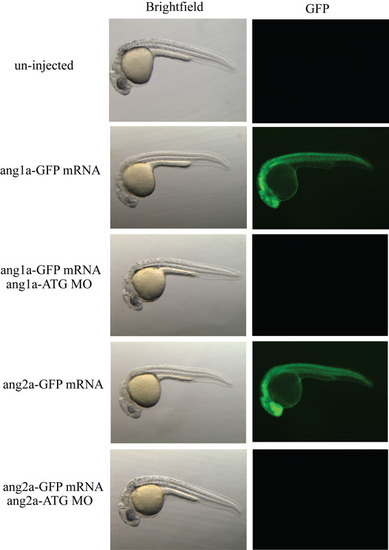
Morpholino specificity controls: MOang1a-ATG and MOang2a-ATG binding sites were cloned into pCS2+, which contained a cassette encoding for GFP, upstream of the translation initiation site. Translation of capped mRNA was efficiently blocked by the respective MOs, demonstrating efficacy of the reagents used. |
|
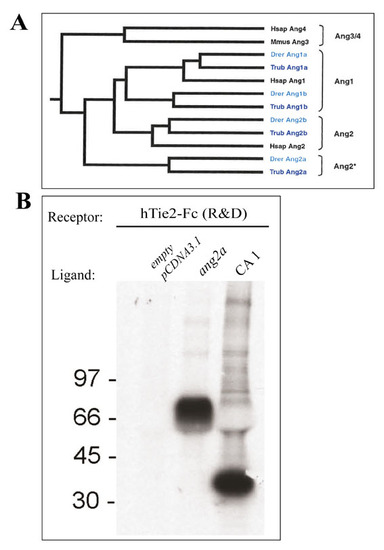
Zebrafish angiopoietin 2a binds to human TIE-2 receptor. (A) A comparison of zebrafish angiopoietin 1 and 2 and their orthologues in Fugu and Human. Clustal W alignment with the protein sequences of zebrafish (Danio rerio, Drer), Fugu-Fish (Takifugu rupripes, Trub) and of human (Homo sapiens, Hsap) shows that zebrafish and fugu-fish angiopoietin 1a and 1b are grouped together with human Angiopoietin 1. Zebrafish and fugu-fish angiopoietin 2b are clustered together with human Angiopoietin 2, whereas zebrafish and fugu-fish angiopoietin 2a is clustered as a separate group. (B) Zebrafish angiopoietin 2a (ang2a) binds to human Tie2-Fc. Full length zf ang2a cDNA was cloned in pCDNA3.1. ang2a, CA1 (Comp-Ang1) or empty pCDNA3.1 as a control were transiently expressed and 35S labelled in 293T cells. hTie2 protein (R&D) were used to pull-down 35S labeled angiopoietins from 293T conditioned medium. The bound proteins were separated on SDS-PAGE, and detected using autoradiography. The results show that hTie2-Fc binds to COMP-Ang1 as well as to Ang2a. |