|
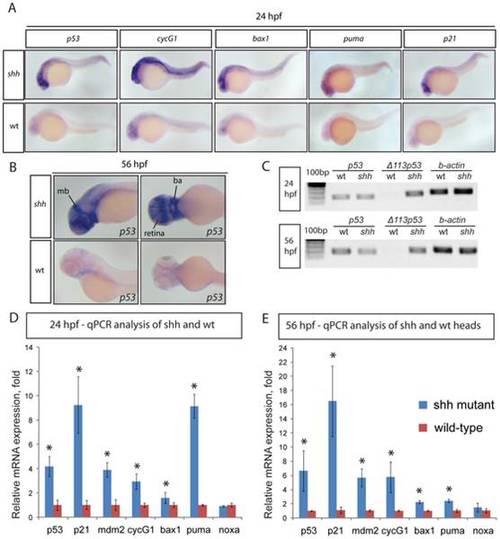
(A) 24 hpf wild-type and shh-/- mutant embryos were in situ hybridised with probes against p53, cyclinG1, bax1, puma and p21 mRNAs. These p53 target genes are expressed at a higher level in the shh-/- mutant in neural tube, retina and somites than in wild-type embryos. (B) 56 hpf wild-type and shh-/- mutant embryos were hybridized with the probe against p53 mRNA. The hybridizations show increased expression of p53 in shh-/- mutant compared to wild-type embryos in the brain, retina and branchial arches. The following structures are labelled: retina, midbrain (mb) and branchial arches (ba). (C) p53 transcript isoforms in shh-/- mutant and wild-type at 24 and 56 hpf were analysed by semi-quantitative PCR assays for full-length p53, Δ113 p53 transcripts and beta;-actin transcript as a loading control. At both stages, full-length p53 and β-actin transcripts were expressed at similar levels in wild-type and mutant samples, whereas Δ113 p53 was strongly upregulated in shh-/- mutant samples. (D, E) qPCR assays for p53, p21, mdm2, cyclinG1, bax1, puma, noxa and gapdh were performed at 24 hpf (D) and 56 hpf (E) on wild-type and shh-/- mutant embryos. cDNA from whole embryos was used for 24 hpf samples and cDNA from embryo heads was used for 56 hpf samples. Gene expression was normalized using gapdh expression as reference. The panels show p53 target gene expression in wild-type and shh-/- mutant embryos (D, E). Error bars represent standard deviation and an asterisk indicates statistical significance. There are significant expression differences between wild-type and shh-/- mutant samples (t-test, * - p<0.05) for all genes except noxa. qPCR assays were performed in triplicates on three different clutches of embryos and standard deviations are shown. |
|
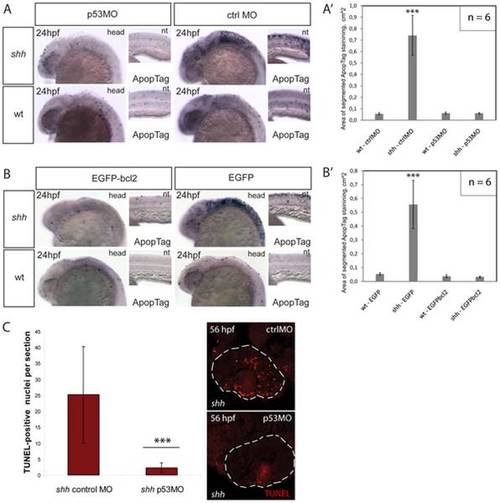
(A) Apoptosis labelling using ApopTag staining in wild-type and shh-/- mutant embryos at 24 hpf after injection of p53 morpholino or control morpholino. The whole-mount images of the head (head) and neural tube (nt) are shown. (A′) Quantification of ApopTag signal area in wild-type and shh-/- mutant embryo head images from (A) shows that p53MO successfully decreased apoptosis in shh-/- mutant to the wild-type level. Asterisks (***) on top of the shh-/- mutant bar indicate its significant statistical differences from other samples (t-test, P-value<0,001). (B) Apoptosis labelling using ApopTag staining in wild-type and shh-/- mutant embryos at 24 hpf after injection of of EGFP-bcl2 or EGFP mRNA. The whole-mount images of the head (head) and neural tube (nt) are shown. (B′) Quantification of ApopTag signal area in wild-type and shh-/- mutant embryo head images from (B) shows that EGFP-bcl2 successfully decreased apoptosis in shh-/- mutant to the wild-type level. Asterisks (***) on top of the shh-/- mutant bar indicate its significant statistical differences from other samples (t-test, P-value<0,001). (C) Retinal apoptosis observed in shh-/- mutant at 56 hpf was suppressed by injection of p53 morpholino but not by injection of control morpholino (n = 10 and 40 sections for shh-/- mutant injected with p53 morpholino; n = 10 and 34 sections for shh-/- mutant injected with control morpholino). *** - t-test, P-value<0,001. |
|
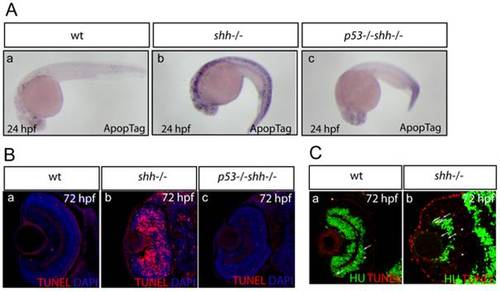
Apoptosis was assayed in whole-mount embryos using ApopTag (A, a-c) and on retinal sections using TUNEL labelling (B, a-c). The sections are oriented with their anterior side to the top. Elevated apoptosis in shh-/- mutant embryos compared to wild-type embryos was suppressed by p53 loss in the developing nervous system (A, a-c) and in the retina (B, a-c). (A) Whole-mount ApopTag staining at 24 hpf of wild-type, shh-/- and p53-/-shh-/- embryos (a-c). (B) TUNEL/DAPI staining of retinal sections of wild-type, shh-/- and p53-/-shh-/- embryos at 72 hpf (a-c). (C) Wild-type (a) and shh-/- mutant (b) retinal cryosections from 72 hpf embryos stained with anti-HU antibodies to detect retinal neurons and TUNEL to label apoptotic cells. Cells positive for both HU and TUNEL are indicated with arrows and cells positive only for TUNEL are labelled with arrowheads. Images were obtained from retinal sections of 10 embryos and representative overlay images are shown. |
|
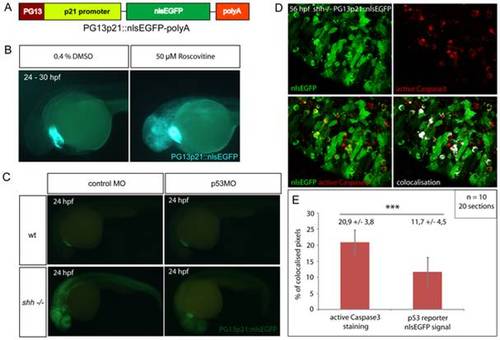
(A) p53 reporter PG13p21::nlsEGFP-polyA was constructed using Tol2kit as described in Materials and methods. It consists of the p21 promoter fused with p53-binding enhancer sequence PG13, nlsEGFP coding sequence and a polyadenylation signal (polyA). (B) Wild-type embryos carrying the p53 reporter transgene were incubated for 6 hours at 24 hpf with either 50 μM Roscovitine or 0.4% DMSO diluted in E3 medium. DMSO-treated embryos did not show any significant expression of the reporter (right), whereas roscovitine-treated ones expressed p53 reporter at a high level (left). All embryos showed green-heart transgenesis marker. The experiment was repeated three times and 20 embryos were used for each treatment. (C) p53 morpholino but not 4-mismatch control morpholino injection suppressed expression of PG13p21::nlsEGFP p53 reporter in shh-/- mutant embryos at 24 hpf. Both injections had no effect on p53 reporter expression in the wild-type embryos. (D) Anti-active Caspase3 staining of cells in the shh-/- mutant retina at 56 hpf expressing p53 reporter transgene PG13p21::nlsEGFP. Most cells positive for active Caspase3 also expressed p53 reporter transgene. Single channel, overlay and co-localisation analysis images are shown. (E) Quantification of co-localisation of active Caspase3 staining and nlsEGFP p53 reporter signal. Co-localised pixels made up 20,9 +/- 3,8% of total active Caspase3 pixels and 11,7 +/ 4,5% of total nlsEGFP pixels. Quantifications were performed on 20 retinal section images from 10 embryos. Asterisks (***) indicate a significant statistical difference between the proportions (t-test, P-value<0,001). |
|
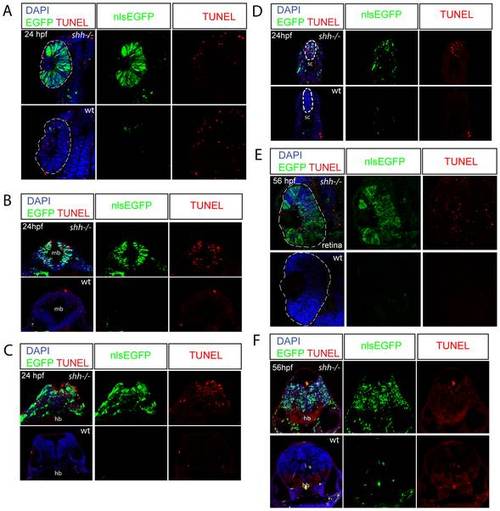
Wild-type and shh-/- embryos at 24hpf (A-D) and 56 hpf (E, F) carrying p53 reporter transgene (PG13p21::nlsEGFP) were transversally sectioned, nuclei were stained with DAPI and apoptotic cells - with TUNEL, nlsEGFP signal is endogeneous (A-F). The developing nervous system of shh-/- mutant embryos showed high level of p53 reporter expression and apoptosis compared to wild-type embryos at both 24 (B-D) and 56 hpf (F). (A) At 24 hpf, p53 reporter was strongly induced in the shh-/- mutant compared to the wild-type retina, but there was no elevated apoptosis in the shh-/- mutant retina. (B) Expression of p53 reporter and TUNEL labeling in midbrain of shh-/- mutant and wild-type embryos at 24 hpf. (C) Expression of p53 reporter and TUNEL labeling in hindbrain of shh-/- mutant and wild-type embryos at 24 hpf. (D) Expression of p53 reporter and TUNEL labelling in spinal cord of shh-/- and wild-type at 24 hpf. (E) At 56 hpf, both p53 reporter expression and apoptosis were much higher in the shh-/- retina than in the wild-type retina. (F) Expression of p53 reporter and TUNEL labelling in hindbrain regions of shh-/- and wild-type embryos at 56 hpf. |
|
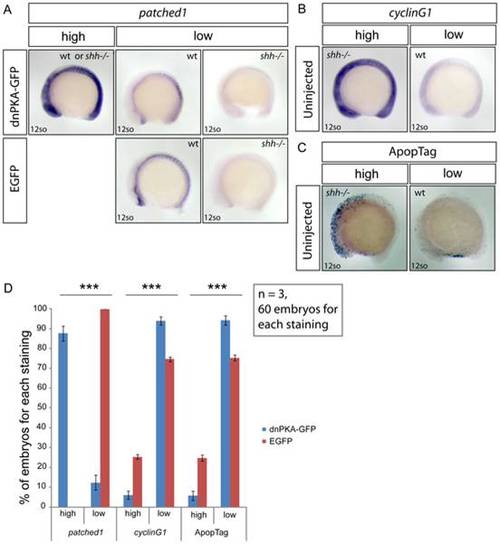
Embryos from shh+/- parent fish were injected with either dnPKA-GFP or EGFP mRNA, grown to 12-somite stage and in situ stained for patched1, target gene of Hh signaling, and cyclinG1, p53 target gene, and ApopTag staining was performed to characterize the level of apoptosis. (A) Injection of dnPKA-GFP led to a strongly increased and more widely spread expression of patched1 (category “high”) in 87,7+/-3,8% of injected embryos, the rest having normal wild-type or low shh-/- mutant patched1 expression comparable to expression of patched1 in EGFP-injected embryos from shh+/- parent fish (category “low”). (B) Expression of cyclinG1 in uninjected embryos from shh+/- parent fish. Categories “high” and “low” indicate expression levels in shh-/- mutant and wild-type embryos, respectively. (C) ApopTag staining of apoptotic cells in uninjected embryos from shh+/- parent fish. Categories “high” and “low” indicate levels of apoptosis in shh-/- mutant and wild-type embryos, respectively. (D) Statistical analysis of results from injections of dnPKA-GFP and EGFP mRNA into embryos from shh+/- parent fish. All of EGFP-injected embryos had “low” expression of patched1, 25,3 +/- 1,15% (Mean +/- standard deviation) of them had “high” expression of cyclinG1 and 24,7 +/- 1,53% had “high” apoptosis levels. dnPKA-GFP mRNA injection led to “high” expression of patched1 in 87,7 +/- 3,8% of embryos and decreased expression of cyclinG1 and apoptosis levels: “high” proportions are 6 +/- 2% and 5,8 +/- 2,4%, respectively. The experiment was done 3 times and 60 embryos were used for each staining. Asterisks (***) on top of shh-/- mutant bars indicate significant statistical differences of proportions (Fisher′s exact test, P-value<0,001). |
|
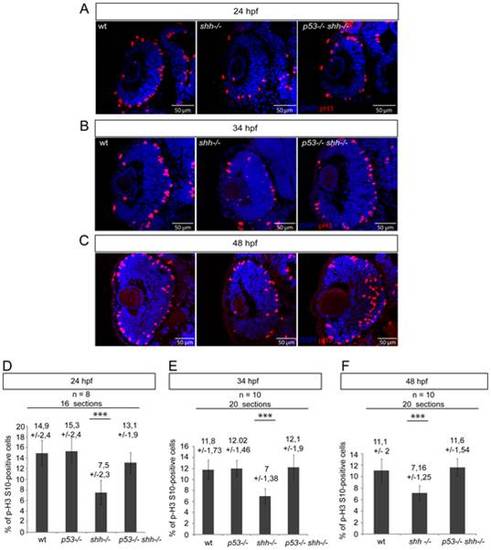
pH 3/DAPI staining of wild-type, shh-/- and p53-/-shh-/- retinas was done at 24 hpf (A), 34 hpf (B) and 48 hpf (C). At all stages, the mitotic index in the shh-/- retina was lower than in the wild-type retina, while in the p53-/-shh-/- retina, the mitotic index was comparable to that of the wild-type retina. (D) Statistical analysis of mitotic indices in wild-type, p53-/-, shh-/- and p53-/-shh-/- retinas at 24 hpf, the indices being comparable in wild-type (14,9%, SD = 2,4%), p53-/- (15,3%, SD = 2,4%), and p53-/-shh-/- (13,1%, SD = 1,9%) embryos and significantly higher than in the shh-/- mutant retina (7,5%, SD = 2,3%). (E) Statistical analysis of mitotic indices in the wild-type, p53-/-, shh-/- and p53-/-shh-/- retinas at 34 hpf, comparable in wild-type (11,8%, SD = 1,73%), p53-/- (12,02%, SD = 1,46%), and p53-/-shh-/- (12,1%, SD = 1,9%) retinas, whereas the mitotic index in the shh-/- mutant retina was almost twice lower (7%, SD = 1,38%). (F) Statistical analysis of mitotic indices in wild-type, shh-/- and p53-/-shh-/- retinas at 48 hpf, the indices being comparable in wild-type (11,1%, SD = 2%) and p53-/-shh-/- (11,6%, SD = 1,54%) embryos and significantly higher than in the shh-/- mutant retina (7,16%, SD = 1,25%). Either 8 (D) or 10 (E, F) embryos (2 retinal sections per embryo) were analysed for each genotype and mean +/- standard deviation indicated above the bars of mitotic index statistics. Asterisks (***) on top of shh-/- mutant bars indicate their significant statistical differences from other samples (t-test, P-value<0,001). |
|
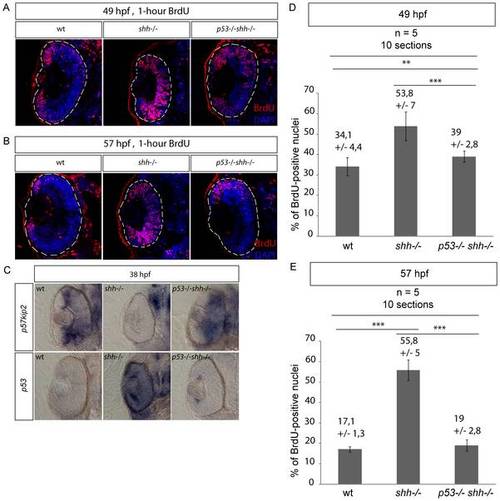
S-phase progression in wild-type, shh-/- and p53-/-shh-/- mutants was analysed at 49 and 57 hpg after one-hour labelling with BrdU. Confocal sections stained with anti-BrdU antibodies and DAPI are shown with the anterior side to the top. (A) Anti-BrdU/DAPI staining of wild-type, shh-/- and p53-/-shh-/- mutant retinas at 49 hpf identified a greater extent of cell-cycle exit (fewer cells labelled) in the wild-type and p53-/-shh-/- mutant than in the shh-/- retina. The data from these stainings were then used for quantification in (D). (B) At 57 hpf in the wild-type retina, BrdU-positive cells were found only in the ciliary marginal zone (CMZ). However, in the shh-/- mutant retina, cells still failed to exit the cell cycle. Like in the wild-type retina, cells of p53-/-shh-/- mutant mostly exited the cell cycle and proliferative cells were located only in the CMZ. (C) At 38 hpf, p57kip2 expression was at a high level in both wild-type and p53-/-shh-/- mutant retinas, whereas no p57kip2 was present in the shh-/- mutant retina. By contrast, p53 expression was high in the shh-/- mutant retina and very low in the wild-type and p53-/-shh-/- mutant retinas. (D) Proportions of BrdU-positive cells (S-phase indices) in wild-type, shh-/- and p53-/-shh-/- retinas at 49 hpf were obtained from confocal sections such as presented in (A). The S-phase indices in wild-type (34,1%, SD = 4,4%) and p53-/-shh-/- (39%, SD = 2,8%) were each significantly lower than in the shh-/- mutant retina (53,8%, SD = 7%) (t-test, P-value<0,001), the difference between them still being significant (t-test, P-value<0,01). (E) S-phase indices in wild-type, shh-/- and p53-/-shh-/- retinas at 57 hpf were obtained from confocal sections such as presented in (B). The S-phase indices in wild-type (17,1%, SD = 1,3%) and p53-/-shh-/- (19%, SD = 2,8%) were not significantly different (t-test, P-value>0,05), but were each significantly lower than in the shh-/- mutant retina (55,8%, SD = 5%) (t-test, P-value<0,001). Total 10 sections from 5 embryos (2 retinal sections per embryo) were analysed for each genotype and mean +/- standard deviation are indicated above the graph bars. Statistical differences were evaluated using t-test with P-values<0,01 (**) or<0,001 (***). |
|
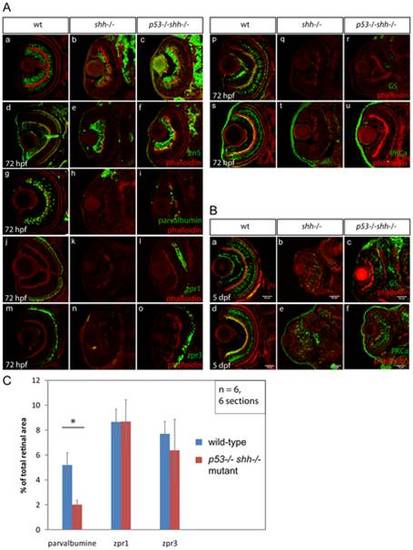
Differentiation of retinal cell types was assessed using specific antibodies (A, B). The sections are oriented with their anterior side to the top. All sections were stained with phalloidin-Alexa568 (A, a–u; B, a–f) to visualize retinal lamination. Staining for HuC neuronal antigen labelled ganglion and amacrine cells in the wild-type retina (A, a), ganglion and very few amacrine cells in the shh-/- mutant retina (A, b). In the p53-/-shh-/- retina, however, labelling of both ganglion cells and amacrine cells was partially restored (A, c). Ganglion cells labelled by an anti-zn5 antibody were present in comparable numbers in wild-type (A, d), shh-/- mutant (A, e) and p53-/-shh-/- mutant retinas (A, f). Amacrine cells labelled by anti-parvalbumine antibody were present in the wild-type retina (A, g), absent in the shh-/- mutant (A, h) and partially rescued in the p53-/-shh-/- mutant retina (A, i). Red-green double cones (zpr1 antibody labelling) and rod photoreceptors (zpr3 antibody labeling) were present in the wild-type retina (A, j, m), absent in the shh-/- mutant retina (A, k, n) and rescued in the p53-/-shh-/- mutant retina (A, l, o). Staining for glutamine synthetase (GS) labelled Müller glia cells in wild-type embryos (A, p). In both shh-/- and p53-/-shh-/- retinas, very few Müller glia were present (A, q, r). Bipolar cells were labelled in the wild-type retina by anti-PKCα antibody staining (A, s), but absent in shh-/- and p53-/-shh-/- retinas (A, t, u). At 5 dpf, Müller glia and bipolar cells were detected in shh-/- and p53-/-shh-/- retinas (B, b,c,e,f) but in lower numbers than in the wild-type retina (B, a,d). All stainings were performed on at least 6 embryos for each genotype and representative images are shown. (C) Quantitation of rescue of amacrine cells (parvalbumine labelling), red-green double cone (zpr1) and rod photoreceptors (zpr3) in the p53-/-shh-/- mutant retina by plotting ratios of segmented area for each staining to the total retina area. Amacrine cells occupy a significantly larger relative area in the wild-type than in the p53-/-shh-/- mutant retina (* on top of the bars, t-test, P-value<0,05), whereas relative areas of both types of photoreceptors are not significantly different in wild-type and p53-/-shh-/- mutant retinas. |