- Title
-
Transgenic zebrafish reveals novel mechanisms of translational control of cyclin B1 mRNA in oocytes
- Authors
- Yasuda, K., Kotani, T., Ota, R., and Yamashita, M.
- Source
- Full text @ Dev. Biol.
|
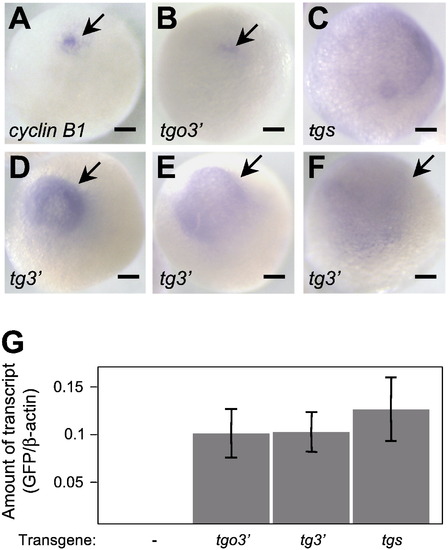
Localization of cyclin B1 and reporter mRNAs in full-grown oocytes. (A–F) Whole-mount in situ hybridization using cyclin B1 (A) and gfp (B–F) probes of full-grown oocytes derived from transgenic fish carrying the tgo3′ (A, B), tgs (C) or tg3′ (D–F) gene. Arrows indicate localized signals. cyclin B1 and tgo3′ mRNAs were localized as an aggregation (A, B), while tgs mRNA was distributed throughout the oocytes (C). tg3′ mRNA was distributed in the hemisphere of oocytes in various patterns, Classes I (D), II (E) and III (F) (see text). Bars indicate 100 μm. (G) Amount of the reporter mRNAs, normalized to that of β-actin mRNA. Real-time PCR using GFP primers showed that the transcripts of three reporter genes are deposited at similar levels in full-grown oocytes. Error bars indicate mean ± standard error of the mean (s.e.m.), n = 3. |
|
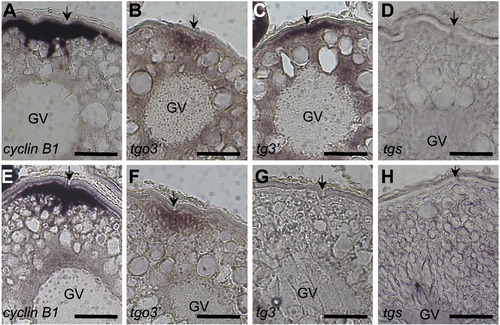
Localization of cyclin B1 and reporter mRNAs during oogenesis. (A–H) Section in situ hybridization using cyclin B1 (A, E) and gfp (B–D, F–H) probes of stage III (A–D) and stage IV (E–H) oocytes derived from wild-type (A, E) and transgenic fish carrying tgo3′ (B, F), tg3′ (C, G) or tgs (D, H) gene. Arrows indicate the micro-pile existing at the animal pole of fish oocytes. cyclin B1 and tgo3′ mRNAs were localized in the cortical cytoplasm beneath the animal pole of stage III (A, B) and IV (E, F) oocytes. tg3′ mRNA was localized to the animal pole of stage III oocytes (C) but disappeared in stage IV oocytes (G). tgs mRNA showed no localization during oogenesis (D, H). GV, germinal vesicle. Bars indicate 100 μm. |
|
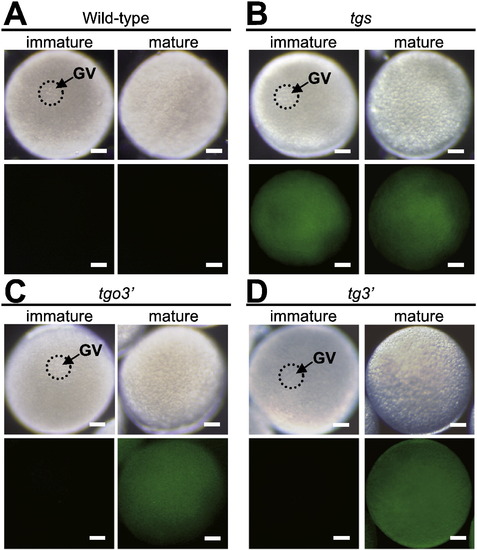
Translational regulation of reporter mRNAs in immature and mature oocytes. (A–D) Immature and mature oocytes derived from wild-type (A) and transgenic fish carrying tgs (B), tgo3′ (C) and tg3′ (D) genes. Bright-field views (upper panels) and GFP fluorescence (lower panels) are shown. No GFP fluorescence was detected in wild-type immature and mature oocytes (A), while GFP fluorescence was observed in immature and mature oocytes expressing the tgs mRNA (B). GFP fluorescence was not detected in immature oocytes but was visible in mature oocytes expressing the tgo3′ and tg3′ mRNAs (C, D). Bars indicate 100 μm. GV, germinal vesicle. |
|
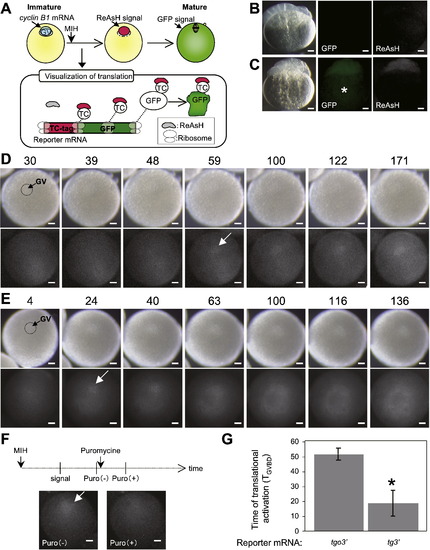
Real-time imaging of temporally controlled translation. (A) Schematic views for visualization of the translation site and timing during oocyte maturation. The cyclin B1 mRNA is localized to the animal pole of immature oocytes (blue circle). After MIH stimulation, the reporter mRNA is translated, which is detected by binding of ReAsH dye to the nascent polypeptide chains of TC-tag. The GFP protein is matured at the later stage. (B, C) Visualization of ReAsH signal in cleavage-stage embryos. Embryos derived from wild-type fish (B) and transgenic fish carrying the tgs gene (C) and treated with ReAsH dye showing the fluorescent ReAsH signal (ReAsH) in embryos expressing TC-tagged GFP (GFP) but not in wild-type embryos. GFP in yolk granule (asterisk) is unable to be detected by ReAsH dye for an unknown reason. (D, E) Real-time imaging of temporally controlled translation of the tgo3′ mRNA (D) and tg3′ mRNA (E). The times after MIH stimulation are shown as standardized time TGVBD (see text). Arrows indicate ReAsH signals detected at the first time after MIH treatment. Translation of tgo3′ mRNA was first detected at the time TGVBD = 59 at the animal pole of the oocyte. The translational site was retained at the animal pole during oocyte maturation (D). In contrast, translation of tg3′ mRNA was first detected at the time TGVBD = 24 at the animal pole of the oocyte. The translational site became widely distributed throughout the hemisphere of the oocyte during oocyte maturation (E). (F) Effects of puromycin treatment on the ReAsH signal. The oocyte expressing tgo3′ mRNA was injected with ReAsH dye and treated with MIH. The ReAsH signal detected after MIH treatment [Puro(-)] was dispersed by puromycin treatment [Puro(+)]. (G) The times that translation of the reporter mRNA occurs after MIH stimulation. Error bars indicate mean ± s.e.m., n = 7 for tgo3′ and n = 4 for tg3′, asterisk, P < 0.05 (Student′s t-test). GV, germinal vesicle. |
|
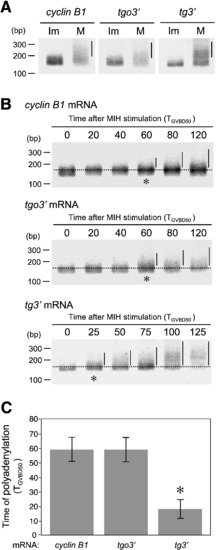
Cytoplasmic polyadenylation of cyclin B1 and reporter mRNAs during oocyte maturation. (A) Cytoplasmic polyadenylation of cyclin B1 (cyclin B1), tgo3′ (tgo3′) and tg3′ (tg3′) mRNAs in immature (Im) and mature (M) oocytes. The poly(A) tails of all mRNAs remained short in immature oocytes and were elongated in mature oocytes. The efficiency of polyadenylation of zebrafish cyclin B1 is lower than that of Xenopus cyclin B1 because of the differences in the 3′ UTR sequences. Bars indicate the elongated poly(A) tail. (B) Time courses of polyadenylation of cyclin B1, tgo3′ and tg3′ mRNAs. Dotted lines indicate the basal size of the poly(A) tails. Asterisks indicate when polyadenylation of each mRNA is initiated. Polyadenylation of cyclin B1 and tgo3′ mRNAs was initiated at the time TGVBD50 = 60, while that of tg3′ mRNA was initiated at TGVBD50 = 25. (C) The times that polyadenylation of cyclin B1 and reporter mRNAs occurs after MIH stimulation. Error bars indicate mean ± s.e.m., n = 3, asterisk, P < 0.05 (Student′s t-test). |
|
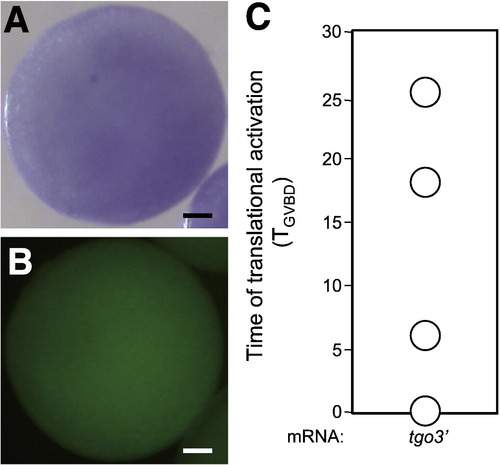
Localization and translational control of in vitro-transcribed mRNA in oocytes. (A) Whole-mount gfp in situ hybridization of a full-grown oocyte fixed 4 h after injection with tgo3′ mRNA. (B) Dextran fluorescence of the full-grown oocyte 4 h after injection. The in vitro-transcribed tgo3′ mRNA and dextran appeared to be diffused after injection. (C) The times when translation of the injected mRNA occurs after MIH stimulation. Translational repression of the tgo3′ mRNA was derepressed before (TGVBD = 0) or after MIH treatment, with timings much earlier than that of the mRNA transcribed in vivo ( Fig. 5G). |
Reprinted from Developmental Biology, 348(1), Yasuda, K., Kotani, T., Ota, R., and Yamashita, M., Transgenic zebrafish reveals novel mechanisms of translational control of cyclin B1 mRNA in oocytes, 76-86, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.