- Title
-
PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury
- Authors
- North, T.E., Babu, I.R., Vedder, L.M., Lord, A.M., Wishnok, J.S., Tannenbaum, S.R., Zon, L.I., and Goessling, W.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
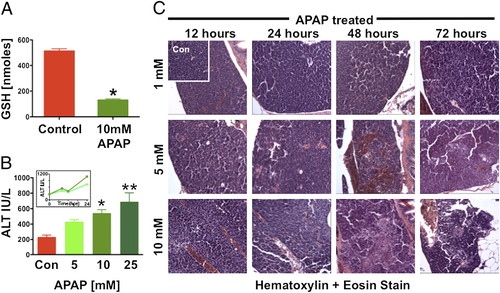
Acetaminophen causes liver toxicity in adult zebrafish. Adult zebrafish were exposed to APAP for 24 h. (A) Total liver GSH was significantly diminished after exposure to 10 mM APAP. *t test, P d 0.001, n e 4. (B) ALT levels were measured 6 hpe to 5, 10, and 25 mM APAP. *Significant vs. control. **Significant vs. 5 mM, ANOVA, P < 0.01, n = 20/sample for three replicates. (Inset) ALT levels measured over time after exposure to 5 and 10 mM APAP rose over the first 24 hpe (n e 10/measurement). (C) Effects of increasing APAP doses on liver histology; hepatocyte necrosis, sinusoidal widening, and focal hemorrhage can be seen. |
|
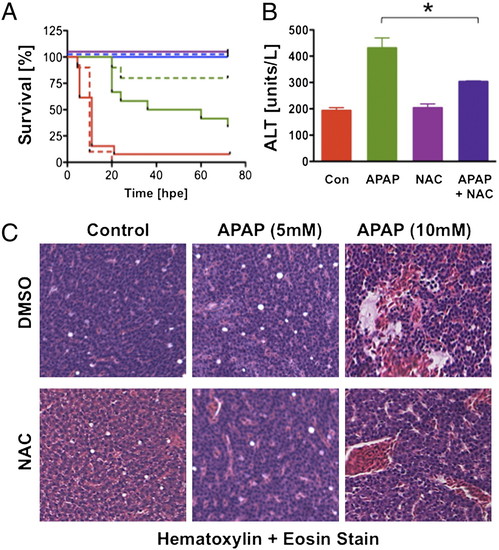
N-acetylcysteine prevents liver toxicity. (A) Survival was assessed in adult zebrafish exposed to 5 (blue), 10 (green), or 25 (red) mM APAP alone (solid line) and with 10 μM NAC (dashed line; n e 10 per group). (B) APAP-induced elevation of ALT is reversed by the addition of NAC. *ANOVA, P < 0.05, 15–20 fish for three replicates). (C) Zebrafish were exposed to APAP and NAC at the indicated doses; NAC decreased the extent of necrosis after APAP. |
|
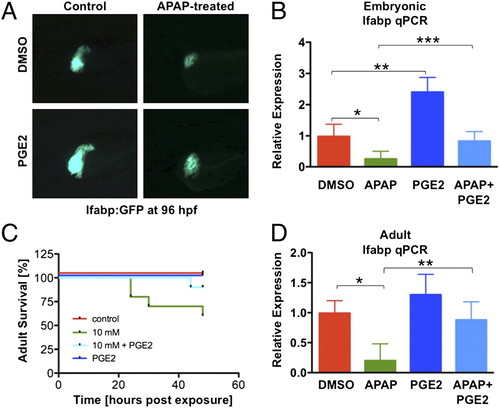
Prostaglandin E2 (PGE2) inhibits APAP toxicity by enhancing proliferation and inhibiting apoptosis. (A) PGE2 (10 μM) increases embryonic liver growth (at 120 hpf) and diminishes 10 mM APAP toxicity in lfabp:GFP embryos. (B) Embryonic lfabp expression at 96 hpf, by qPCR, after the indicated treatment (ANOVA, n = 5). *P = 0.06. **P < 0.001. ***P = 0.025. (C) Survival in adult zebrafish (n = 10/treatment) after APAP is improved by dmPGE2. (D) lfabp expression in the adult liver, by qPCR, is reduced by APAP and rescued by dmPGE2 (ANOVA, n = 4). *P = 0.002. **P = 0.0057. |
|
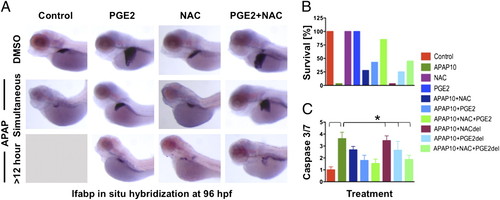
PGE2 acts synergistically with NAC to improve APAP toxicity. (A) Zebrafish embryos were treated with APAP at 48 hpf and with dmPGE2 and/or NAC, either concomitantly or with 12-h delay (del). Combined NAC and PGE2 treatment improved liver morphology, assessed by in situ hybridization for lfabp at 96 hpf, even after delayed exposure. (B) Embryo survival is dependent on treatment parameter and is improved by NAC and PGE2 exposure. (C) Caspase 3/7 activity, as indicator of apoptosis, was measured in a luminometric assay and normalized to controls. *ANOVA, n = 10/ treatment for three replicates, P < 0.001. |
|
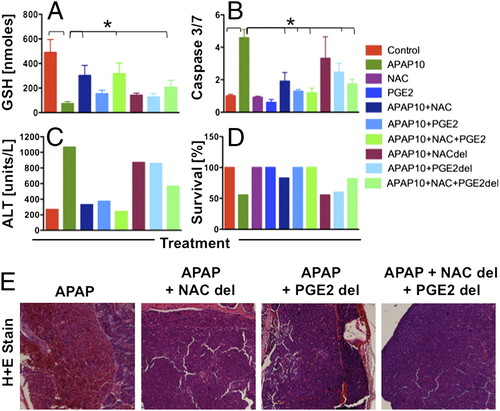
Synergy between NAC and PGE2 limits toxicity and extends the therapeutic window in adult zebrafish after APAP. Adult zebrafish were exposed to APAP and NAC, dmPGE2, or a combination either concomitantly or with 18 h del. (A) GSH (nmoles/whole liver) was improved by combined exposure to NAC and dmPGE2. *ANOVA, n = 3–4, P < 0.01. (B) Relative caspase 3/7 levels were elevated after APAP and improved with immediate NAC and/or dmPGE2; delayed combinatorial therapy was still beneficial. *ANOVA, n = 4–5, P < 0.001. (C) Serum ALT levels (n = 10–20 fish/treatment at 24 hpe) show that combined NAC and dmPGE2 prevented hepatocyte damage by APAP, even after delay. (D) Combined NAC and dmPGE2 treatment led to improved adult zebrafish survival (n = 9–10/treatment) at 48 hpe. (E) Effect of delayed treatment on zebrafish liver histology at 24 hpe; sinusoidal hemorrhage and necrosis in APAP-treated adults could not be reversed with NAC or dmPGE2 alone but only with combined treatment. |
|
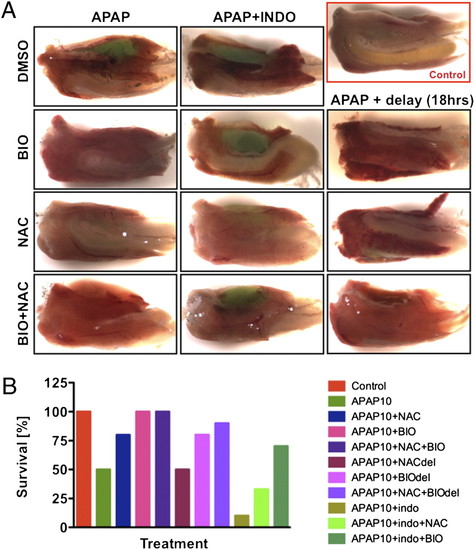
Survival after APAP exposure is affected by wnt and PGE2 modulation. (A) Effect of wnt modulation on adult liver morphology and survival after APAP. Wild-type zebrafish were exposed to APAP and 6-bromoindirubin-32-oxime (BIO) (0.5 μM) and/or NAC (10 μM), either concomitantly or with 18-h delay; BIO prevented liver hemorrhage and improved survival, particularly after delayed treatment. (B) wt adult zebrafish were exposed to 10 mM APAP and indomethacin (10 μM), NAC (10 μM), and BIO (0.5 μM). BIO improved survival if given immediately, either alone or with NAC. If given with 18-h delay (del), the combination of BIO and NAC acted synergistically. Indomethacin in combination with APAP was detrimental to survival, which was improved more substantially by BIO than by NAC. |
|
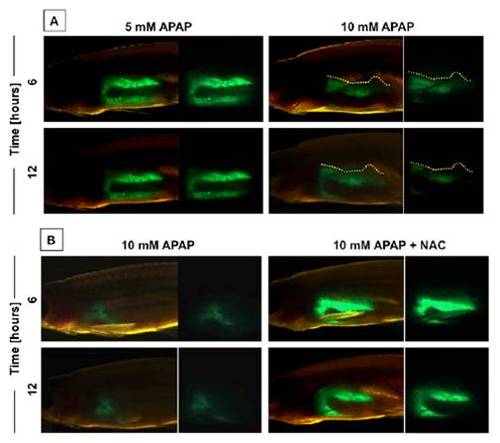
In vivo imaging of APAP injury. (A) In vivo visualization of the effects of 10 mM APAP on liver morphology in adult lfabp:GFP zebrafish at 6 and 12 h postexposure (hpe). (B) In vivo visualization of the effects of APAP and N-acetylcysteine (NAC) on liver morphology. |
|
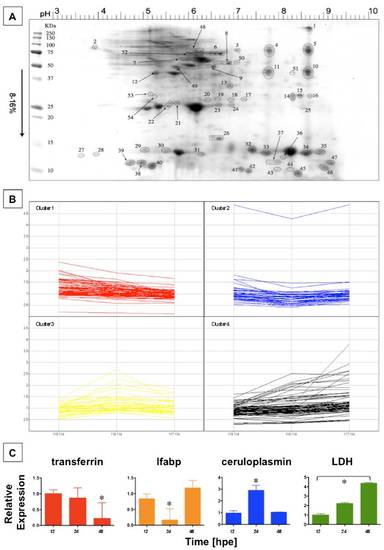
Effect of APAP treatment on serum protein concentration by iTRAQ analysis. (A) Zebrafish serum was collected at 12, 24, and 48 h post-APAP (5 mM) exposure. SDS page revealed 54 spots excised manually from the Coomassie stained gel that were subjected to iTRAQ analysis. (B) The individual relative protein-concentration changes over time are depicted for all four clusters identified. (C) Quantitative PCR (qPCR) was performed for genes representing each cluster on dissected liver samples at 12, 24, and 48 hpe (ANOVA, n = 4). transferrin expression declined over the entire time course. *Significant vs. 12 and 24 hpe, P < 0.05. lfabp and ceruloplasmin expression changed in a biphasic manner. *Significant vs. 12 and 48 hpe, P < 0.01. Lactate dehydrogenase (LDH) expression increased over time. *All time points significant, P < 0.001. |
|
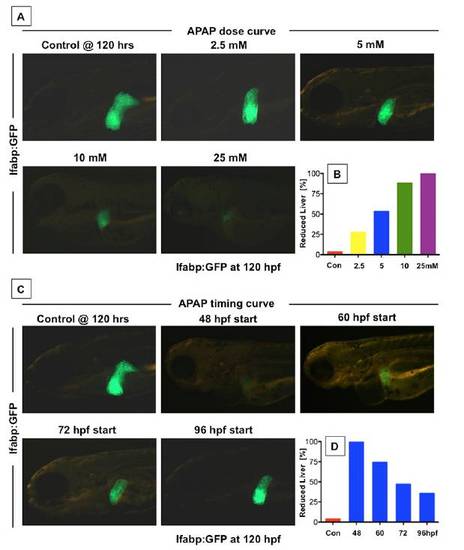
APAP toxicity is dose- and time-dependent in zebrafish embryos. (A) lfabp:GFP transgenic embryos exposed to increasing doses of APAP at 72 hpf and assessed at 120 hpf showed progressively decreasing liver size. (B) Graphical representation of the fraction of zebrafish with diminished liver size at increasing APAP doses (n e 20 embryos for three replicates). (C) lfabp:GFP transgenic embryos were exposed to APAP, and in vivo fluorescence was assessed at 120 hpf. Embryos were exposed to 10 mM APAP at different time points during development. Decreasing time of exposure to APAP caused increasing liver fluorescence and liver size. (D) The fraction of zebrafish with decreased liver size declines with decreasing exposure time; n > 20 embryos/group with three repeats per treatment group. |
|
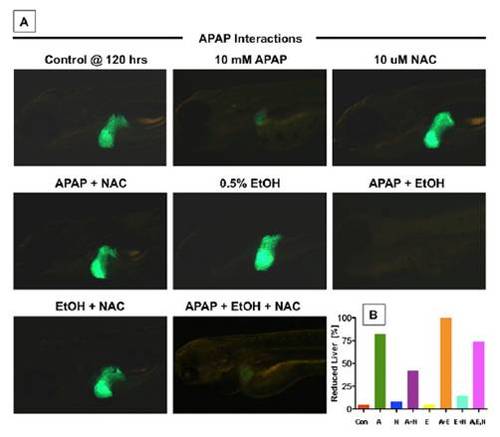
The interaction between APAP, NAC, and EtOH is conserved in zebrafish embryos. (A) Transgenic lfabp:GFP liver reporter zebrafish embryos were exposed to APAP (10 mM), NAC (10 mM), and 0.5% EtOH as indicated. Fluorescence microscopy at 120 hpf revealed the beneficial effect of NAC and the detrimental outcome of combined APAP and EtOH exposures. NAC can rescue the effects of APAP. Short-term EtOH exposure increases liver size. EtOH exerts synergistic liver toxicity with APAP, which cannot be reversed by NAC. (B) Schematic representation of the fraction of zebrafish with decreased liver size, analyzed by in situ hybridization for lfabp. |
|
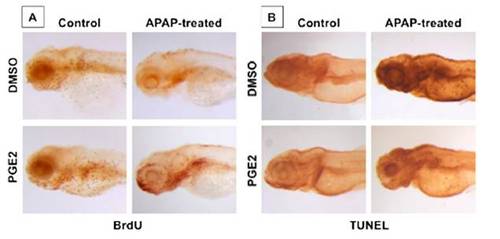
PGE2 inhibits APAP toxicity by enhancing proliferation and inhibiting apoptosis. (A) Embryos were processed for BrdU at 96 hpf following APAP and/ or dmPGE2 (10 μM) treatment. (B) APAP induces liver-specific and global necrosis, by TUNEL staining, that is diminished by dmPGE2 (10 μM). |
|
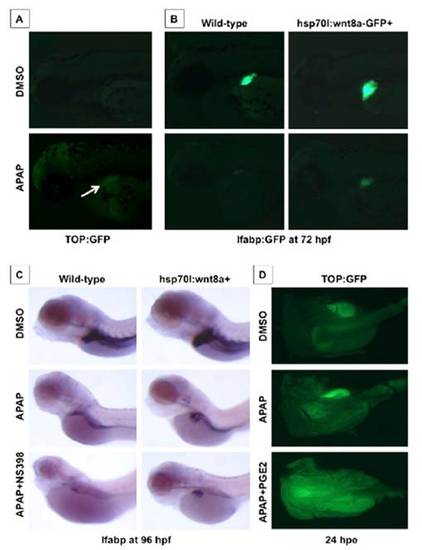
Prostaglandin production influences the effects of wnt8a activation on APAP liver toxicity. (A) Effect of APAP from 48–72 hpf on wnt activity in TOP:dGFP wnt reporter line; APAP caused wnt activation specifically in the embryonic liver. (B) Transgenic Tg(hsp70l:wnt8a-GFP)w34; lfabp:GFP embryos heat-shocked at 48 hpf were exposed to APAP; wnt8a induction rescued liver-specific GFP expression after APAP. (C) Transgenic Tg(hsp70l:wnt8a-GFP)w34 embryos were heat-shocked at 38 °C for 20 min at 48 hpf and exposed to 10 mM APAP; in situ hybridization for lfabp was performed at 96 hpf. wnt8a induction enlarged the liver in untreated embryos and rescued the liver after APAP exposure. Inhibition of PGE2 production by the cox2 inhibitor NS398 (10 μM) enhanced APAP toxicity and diminished the positive effects of wnt8 activation. (D) Adult TOP:GFP wnt reporter fish were exposed to APAP (10 mM) alone or concomitantly to dmPGE2 (10 μM). After 24 h, fish were sacrificed, and the livers were dissected and imaged using a fluorescent dissecting microscope. There is no detectable baseline wnt activity in the untreated liver. APAP exposure enhances wnt activity, which is further increased after additional dmPGE2 exposure. |