- Title
-
Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development
- Authors
- Eberhart, J.K., Swartz, M.E., Crump, J.G., and Kimmel, C.B.
- Source
- Full text @ Development
|
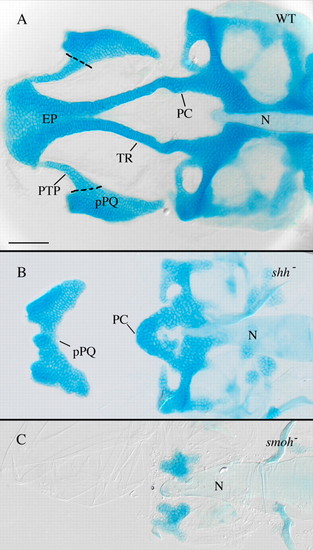
Anterior craniofacial cartilages are especially sensitive to loss of Hh signaling. (A) Flat-mounted 4 dpf wild-type anterior craniofacial cartilages. The anterior neurocranium consists of the trabeculae (TR) and the ethmoid plate (EP). The polar cartilages (PC) fuse with TR joining the anterior and more posterior neurocranium. The dorsal first arch cartilage palatoquadrate (pPQ) and its pterygoid process (PTP) are flat mounted with the neurocranium. (B) Hypomorphic shh- embryos have variable anterior neurocranium defects, including loss of anterior neurocranium and PTP with fusion of pPQ across the midline. (C) Loss of Hh signaling in smo- embryos eliminates most neurocranium cartilages obscuring analysis of defects specific to the anterior neurocranium. N, notochord; WT, wild type. Dorsal views, anterior is leftwards in all panels. Scale bar: 50 μm. |
|
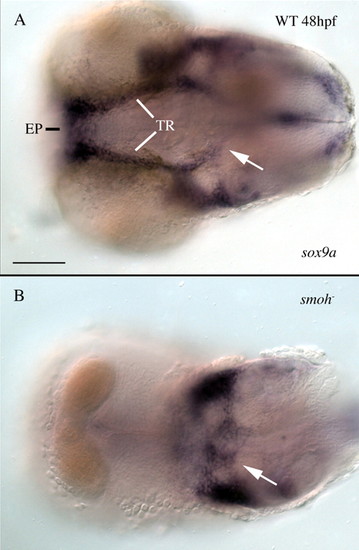
smo- mutant embryos have no anterior neurocranium precartilage condensations. Embryos were stained with RNA probe to the precartilage marker sox9a, the pharyngeal arches were dissected away from the embryos and ventral views of the neurocranium were imaged. (A) At 48 hpf, sox9a-positive cells prefigure the morphology of the 4 dpf neurocranium in wild-type embryos. (B) Embryos lacking all Hh signaling have a complete loss of anterior neurocranium precartilage condensations; however, immediately posterior precartilage condensations are present (arrows in A,B). EP, ethmoid plate; TR, trabeculae; WT, wild type. Anterior is leftwards. Scale bar: 50 μm. |
|
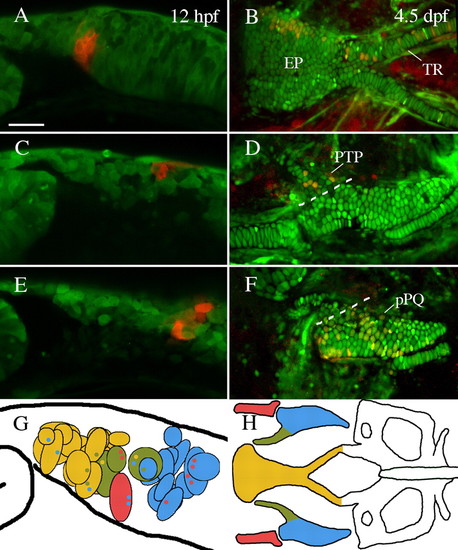
The anterior neurocranium of zebrafish is neural crest-derived. (A,C,E) Lateral views of premigratory crest labeled via photoconversion of Kaede at 12 hpf. (B,D,F) Embryos reimaged at 4.5-5 dpf. (A,B) Cells labeled just posterior to the eye contributed to the ethmoid plate (EP) and trabeculae (TR). (C,D) Neural crest cells slightly more posterior contributed to pterygoid process of the palatoquadrate (PTP). (E,F) Premigratory non-pterygoid palatoquadrate (pPQ) precursors were the most posteriorly localized first arch derivative. (G,H) Schematic representations of fate mapping results show an anteroposterior bias in first arch cartilage elements. Premigratory anterior neurocranium (n=18) and pterygoid process (n=3) precursors (yellow and olive, respectively) are localized more anteriorly than Meckel′s cartilage (MC) (n=1) and palatoquadrate (n=11) precursors (red and blue, respectively). Black outlined circles represent individual labeled embryos. Small colored dots within a field represent secondary cartilage fates from the field of cells. (A,C-G) Lateral views. (B,H) Dorsal views. Anterior is leftwards in all panels. Scale bar: 50 μm. |
|
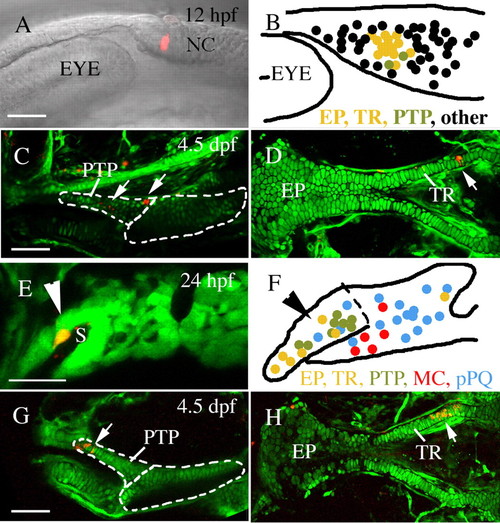
Pre- and postmigratory anterior neurocranium and upper jaw precursors co-localize. Single or two adjacent neural crest cells were labeled via microelectroporation at 12 hpf (A-D) or 24 hpf (E-H, Meckel′s cartilage and palatoquadrate data reanalyzed from Crump et al. unpublished). (A-D) At 12 hpf, pterygoid process (C, arrows, n=2) and anterior neurocranium (D, arrow, n=14) precursors are localized to a discrete cluster postoptically (B). (E-H) Within the first arch, at 24 hpf, pterygoid process (G, arrow, n=8) and anterior neurocranium (H, arrow, n=7) progenitors co-mingle on the stomodeal roof, away from Meckel′s cartilage (n=5) and palatoquadrate (n=16) precursors. The condensed anterior craniofacial neural crest precursor domain is indicated by arrowheads in E and F. (B,F) Schematic enlargements of A and E showing compiled fate mapping data for 12 hpf (B) and 24 hpf (F). Anterior neurocranium, yellow; pterygoid process, olive; non-pterygoid palatoquadrate, blue; Meckel′s cartilage, red. Black dots in B represent fates other than 4.5 dpf skeletal elements. EP, ethmoid plate; MC, Meckel′s cartilage; NC, neural crest; pPQ, non-pterygoid palatoquadrate; PTP, pterygoid process of the palatoquadrate; TR, trabeculae. Anterior is leftwards in all panels. (A,B,D-F,H) Dorsal is upwards. (C,G) Dorsal views. Scale bar: 50 μm. |
|
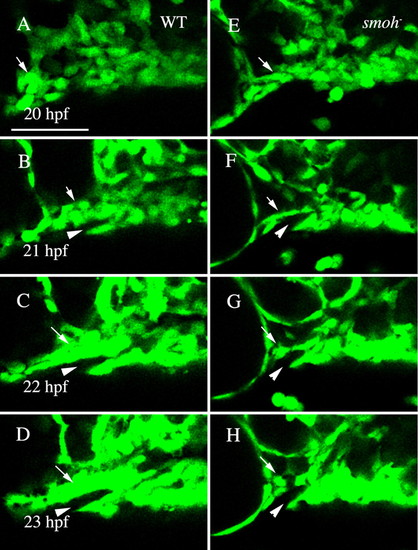
Neural crest cells fail to condense upon the stomodeal roof in smo- embryos. (A-D) Wild-type fli1:GFP embryo. Movie frames are shown every hour from 20 to 23 hpf. Wild-type crest cells are condensing on the stomodeum by 22 hpf (C, arrow) and by 23 hpf (D, arrow) a solid mass surrounding the stomodeum (arrowhead) has formed. (E-H) Neural crest cell behavior in smo- embryos. Crest cells are present just posterior to the eye in smo-;fli1:GFP at 20 hpf (E, arrow). However, these cells fail to condense on the roof of the stomodeum (F-H, arrow). Lateral views, dorsal is upwards. WT, wild type. Scale bar: 50 μm. |
|
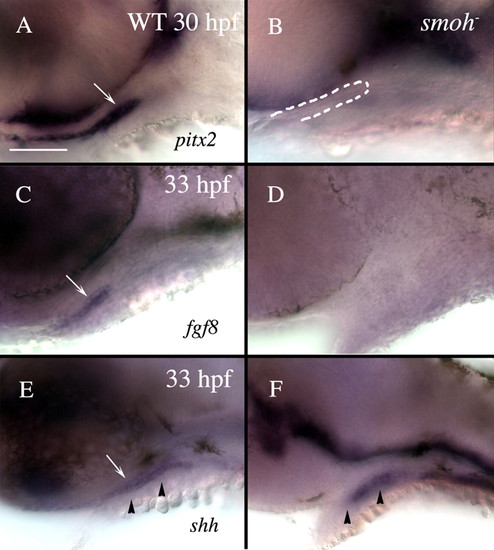
Loss of Hh function causes loss of stomodeum specification. Lateral views of 30 hpf (A,B) or 33 hpf (C-F) wild-type (A,C,E) or smo- (B,D,F) embryos labeled with RNA probe to pitx2 (A,B), fgf8 (C,D) or shh (E,F). (A) Wild-type embryos strongly express pitx2 throughout the stomodeum (arrow), while (B) smo- embryos have no detectable pitx2 expression in the stomodeum, although a morphologically identifiable stomodeum is present (outlined in B). (C) fgf8 labels the lateral stomodeum in wild-type embryos (arrow). (D) No fgf8 expression is detectable in the stomodeum of smo- embryos. (E) shh labels wild-type medial stomodeum (arrow). (F) However, shh is not evident in the stomodeum of smo- embryos, although some medial cells, presumably endoderm, maintain shh expression. Arrowheads in E and F indicate the level of sections shown in Fig. S3. Dorsal is upwards. WT, wild type. Scale bar: 50 μm. |
|
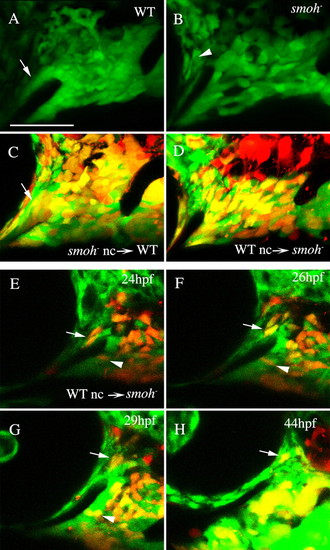
Reception of Hh signaling is not required in neural crest cells to condense on the stomodeum. (A) A tight neural crest cell condensation coats the stomodeum in wild-type 30 hpf fli1:GFP embryos (arrow). (B) The region of this condensation superior to the stomodeum is absent in smo-;fli1:GFP embryos, although GFP-positive sclera is present surrounding the eye (arrowhead). (C,D) 30 hpf embryos following transplantation between wild-type and smo- embryos. (C) smo- neural crest cells readily condense on the stomodeal roof in wild-type embryos (arrow, n=12). (D) Crest cells from wild-type donors fail to condense on the stomodeal roof in smo- embryos, even though they can populate the region occupied by palatoquadrate and Meckel′s cartilage precursors (n=18). (E-H) Images taken from a time-lapse recording (n=2) of wild-type crest in a smo- host. Wild-type neural crest cells are capable of initially populating the region superior to the stomodeum (E-H, arrow); however, these cells are not stabilized in this position and eventually migrate posterior to the eye. Wild-type cells in the Meckel′s cartilage domain condense normally (E-G, arrowhead). Lateral views, dorsal is upwards. nc, neural crest; WT, wild type. Scale bar: 50 μm. |
|
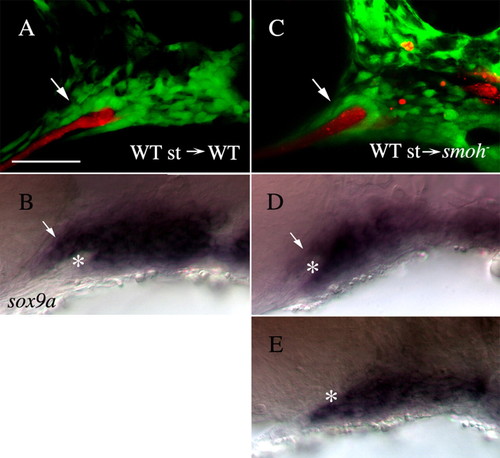
Wild-type stomodeum rescues the condensation of anterior craniofacial neural crest cells in smo- embryos. (A,C) Lateral views of fli1:GFP (A) or smo-;fli1:GFP (C) embryos at 30 hpf following transplantation of wild-type stomodeum. (A) Neural crest cells condense on the stomodeal roof following control transplants (arrow). (C) Following transplantation of wild-type stomodeum into smo-, crest cells condense on the roof of the stomodeum, in a manner similar to that seen in wild type (arrow, n=9). (B,D,E) Lateral views of the same embryos in A,C labeled with RNA probe to sox9a. (B) sox9a-positive cells (arrow) are clearly visible above the stomodeum (*) in control transplanted embryos. (D) sox9a-expressing neural crest cells (arrow) are readily apparent superior to the stomodeum (*) on the side of the embryo receiving the stomodeal transplant. (E) Control, non-transplanted, side of the same embryo as B (image flipped to be the same orientation as that in D). No sox9a-positive cells are observed above the stomodeum (*). Anterior is leftwards. st, stomodeum; WT, wild type. Scale bar: 50 μm. |
|
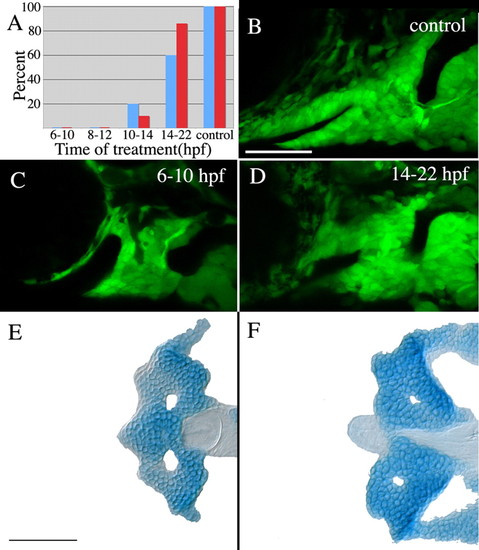
Hh signaling is required at the end of gastrulation for stomodeum expression ofpitx2and proper crest cell condensation. (A) No embryos treated with cyclopamine from 6-10 hpf or 8-12 hpf express stomodeal pitx2 (blue bars) or condense crest on the stomodeal roof (red bars). A small number of embryos treated with cyclopamine from 10-14 hpf express pitx2 in the stomodeum and exhibit condensation of anterior craniofacial crest cells, whereas the majority of embryos treated between 14-22 hpf do express pitx2 in the stomodeum and condense crest cells on the roof of the stomodeum. Control DMSO-treated embryos all expressed pitx2 and undergo crest cell condensation normally. (B-D) Effects of cyclopamine treatment on the condensation of neural crest cells. Anterior craniofacial crest cells condense on the stomodeal roof in control embryos (B) and embryos treated from 14-22 hpf (D); however, this crest cell subpopulation fails to condense in embryos treated from 6-10 hpf (C). (E,F) The anterior neurocranium is deleted in all cyclopamine-treated embryos. n=10 in each treatment group for pitx2 expression. Crest cell condensation and neurocranial cartilage analysis: 6-10 hpf, n=25; 8-12 hpf, n=16; 10-14 hpf, n=20; 14-22 hpf, n=21; control, n=14. Scale bars: 50 μm. |
|
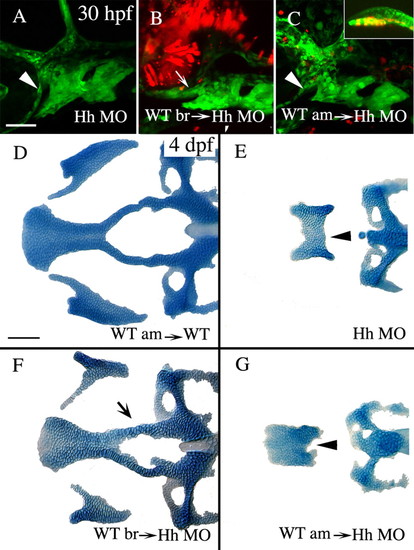
Hh secreted from the ventral presumptive brain, and not the axial mesoderm, is required for condensation of crest cells on the roof of the stomodeum and the subsequent formation of the anterior craniofacial skeleton. (A-C) Lateral views of 30 hpf fli1:GFP embryos co-injected with shh and twhh morpholinos (Hh MO). (D-G) Dorsal views of Alcian stained 4 dpf neurocrania and palatoquadrates from control transplanted embryos (D) or embryos co-injected with Hh MO (E-G). (A,E) Co-injection of Hh MO causes failure of crest cells to condense on the stomodeal roof (A, arrowhead) and the development of the derivatives from this region, the anterior neurocranium and pterygoid process (E, arrowhead; skeletal index=0.1, n=57). (B,F) Transplantation of wild-type brain into Hh MO-injected embryos is capable of rescuing the condensation of neural crest cells (B, arrow) and the skeletal derivatives almost to wild-type morphology (F, arrow, skeletal index=2.2, n=40, ANOVA F ratio=64.481, P<0.0001, Tukey-Kramer shows significance is only attributable to wild-type brain transplant). (C,G) Transplantation of axial mesoderm, including prechordal plate (C, inset), into Hh MO injected embryos has no effect on crest cell condensation (C, arrowhead) or on anterior neurocranium and upper jaw cartilage morphology (G, arrowhead, skeletal index=0.2, n=14). Anterior is leftwards. am, axial mesoderm; br, brain; WT, wild type. Scale bar: 50 μm. |
|
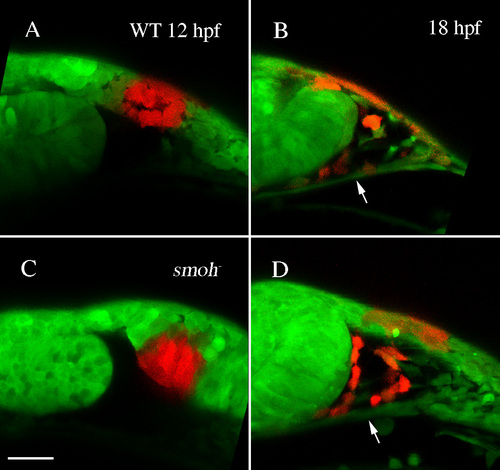
Neural crest cells migrate superior to the stomodeum in smo- embryos. (A,C) Kaede protein was photoconverted at 12 hpf in wild-type (A) or smoh- (C) embryos. (B,D) At 18 hpf, neural crest cells had migrated dorsal to the stomodeum (arrow) in both wild-type (B) and smo- (D) embryos. |
|
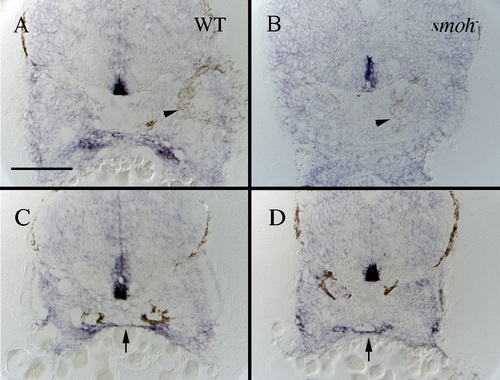
Expression of shh is maintained in presumptive endoderm but not presumptive stomodeum of smoh-embryos. (A,B) Cross-sections taken at the posterior edge of the eye (arrowhead) show shh is expressed in presumptive stomodeum of wild-type (A, arrow), but not smoh- (B) embryos. (C,D) More posterior sections, still through the first arch, show shh is expressed by presumptive medial endoderm in both wild-type (C, arrow) and smoh- (D, arrow) embryos. |
|
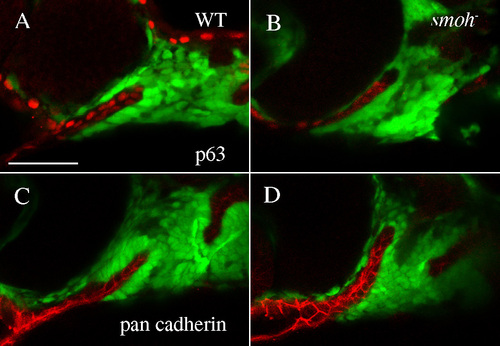
Epithelial integrity of the stomodeum is intact in smo- embryos. Epithelial markers p63 (A,B) and pan-cadherin (C,D) are expressed in the stomodeum of both wild-type (A,C) and smo- embryos (B,D). |
|
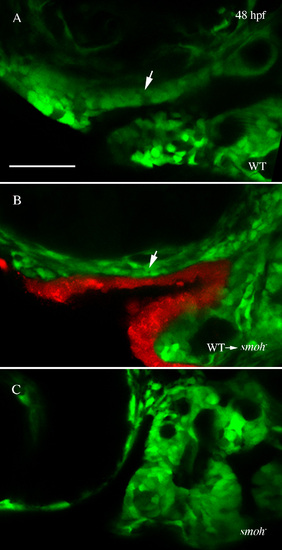
Rescue of anterior craniofacial outgrowth by wild-type stomodeum in smo- embryos. (A,B) Confocal images show outgrowth of the developing anterior craniofacial domain (arrow) in 48 hpf wild-type (A) and smo- embryos receiving wild-type stomodeum transplants (B). (C) No outgrowth is evident in smo- embryos not receiving transplants. |