- Title
-
Negative Feedback Regulation of Wnt4 Signaling by EAF1 and EAF2/U19
- Authors
- Wan, X., Ji, W., Mei, X., Zhou, J., Liu, J.X., Fang, C., and Xiao, W.
- Source
- Full text @ PLoS One
|
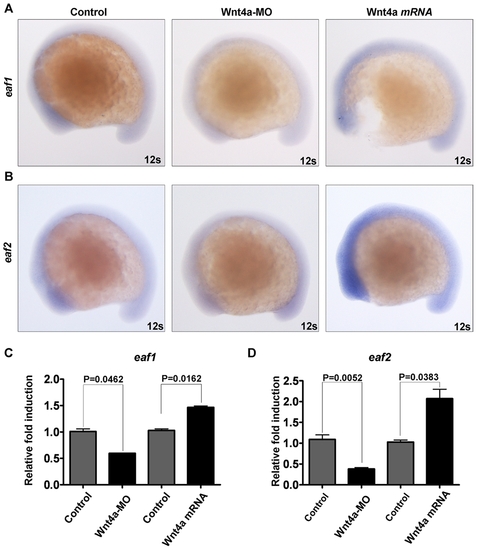
Both zebrafish eaf1 and eaf2/u19 genes are downstream factors of zebrafish Wnt4a. (A) Zebrafish embryos were injected with Wnt4-MO and wnt4a mRNA and the levels of zebrafish eaf1 mRNA determined by whole-mount in situ hybridization at 12-somite stage. (B) Zebrafish embryos were injected with Wnt4-MO and wnt4a mRNA and the levels of zebrafish eaf/u19 mRNA determined by whole-mount in situ hybridization. 12s, 12 somites. (C) Semi-quantitative RT-PCR analysis of eaf1 expression in zebrafish embryos (12 somites) injected with Wnt4a-MO and wnt4a mRNA. (D) Semi-quantitative RT-PCR analysis of eaf2/u19 expression in zebrafish embryos (12 somites) injected with Wnt4a-MO and wnt4a mRNA. EXPRESSION / LABELING:
|
|
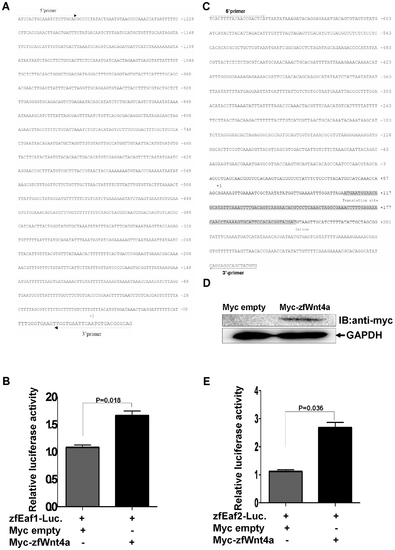
Zebrafish wnt4a can activate the zebrafish eaf1 and eaf2/U19 promoters. (A) The sequence of the 1.2 kb zebrafish eaf1 promoter and the positions of primers used for subcloning. The transcriptional initial site is indicated by +1. (B) Zebrafish embryos were co-injected with the eaf1 promoter reporter and either a control vector or a vector expressing Myc-Want4a. The luciferase activity was normalized to Renilla and reported as the mean ± standard deviation (SD). (C) The sequence of the 1.0 kb zebrafish eaf2/u19 promoter and the primers used for subcloning. The transcriptional initial site is indicated by +1. (D) The expression of Myc-Wnt4a was verified by Western blot using an anti-Myc antibody. (E) Zebrafish embryos were co-injected with the eaf2/u19 promoter reporter and either a control vector or a vector expressing Myc-Wnt4a. The luciferase activity was normalized to Renilla and reported as the mean ± SD. |
|
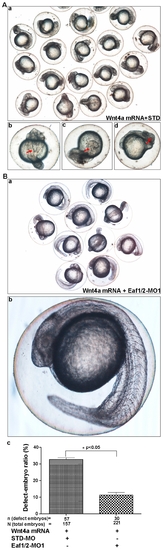
The knockdown of zebrafish Eaf1 and Eaf2/U19 rescues the embryonic defects caused by wnt4a over-expression. (A) Zebrafish embryos were co-injected with wnt4a mRNA (10 pg) and standard morpholino (STD) (8 ng). Defects, indicated by red arrows, included (b) folking notochord, (c) cyclopia, and (d) shortened axis. (B) (a) The embryos were co-injected with wnt4a mRNA and Eaf1/2-MO1to rescue the phenotype resembles wild-type embryos. (b) An example of a rescued embryo. (c) The efficiency of the Eaf1/Eaf2-MO1-mediated rescue is statistically significant (p<0.05) by counting defective embryos. |
|
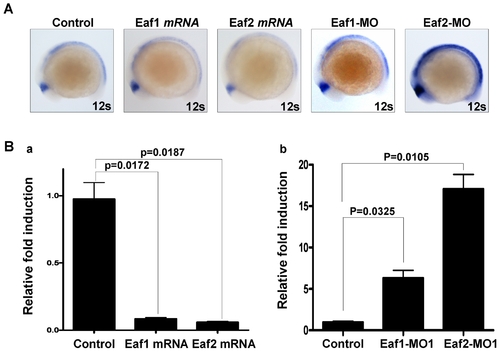
Both zebrafish eaf1 and eaf2/u19 suppress zebrafish wnt4a expression. (A) Whole-mount in situ hybridization analysis of zebrafish wnt4a expression in embryos injected with eaf1 mRNA, eaf2/u19 mRNA, Eaf1-MO and Eaf2-MO at 12-somite stage. The embryos without injection were used as control. (B) The expression of zebrafish wnt4a mRNA was suppressed by ectopic expression of zebrafish eaf1 and eaf2/u19 (a) and up-regulated by knockdown Eaf1 and Eaf2/U19 (b) as revealed by semi-quantitative RT-PCR analysis at 12-somte stage. EXPRESSION / LABELING:
|
|
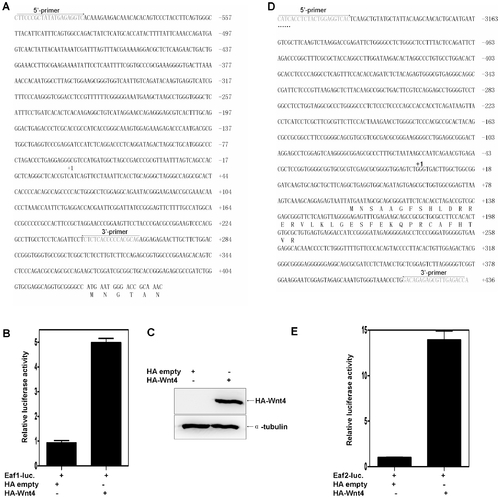
Both human EAF2/U19 and EAF1 are Wnt4 downstream factors. (A) The sequence of the 1.0 kb human EAF1 promoter and the positions of primers used for subcloning. The transcriptional initial site is indicated by +1. (B) 293 cells were co-transfected with the EAF1 promoter reporter and either a control vector or a vector expressing HA-Wnt4. The luciferase activity was normalized to Renilla and reported as the mean ± standard deviation (SD). (C) The expression of Wnt4 was verified by Western blot using an anti-HA antibody. (D) The partial sequence of the 3.6 kb human EAF2/U19 promoter and the primers used for subcloning. The transcriptional initial site is indicated by +1. (E) .293 cells were co-transfected with the EAF2/U19 promoter reporter and either a control vector or a vector expressing HA-Wnt4. The luciferase activity was normalized to Renilla and reported as the mean ± SD. |
|
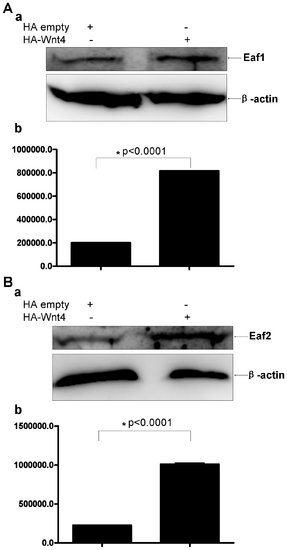
Western blot analysis of human endogenous EAF1 and EAF2/U19 protein expression after the introduction of ectopic Wnt4 expression in 293 cells. (A) Representative Western blot of EAF1 in 293 cells transfected with a control vector or a vector expressing Wnt4 using a polyclonal antibody against human EAF1. (b) EAF1 expression was normalized to β-actin. (B) Representative Western blot of EAF2/U19 in 293 cells transfected with a control vector or a vector expressing Wnt4 using a polyclonal antibody against human EAF2/U19. (b) EAF2/U19 expression normalized to β-actin. |
|
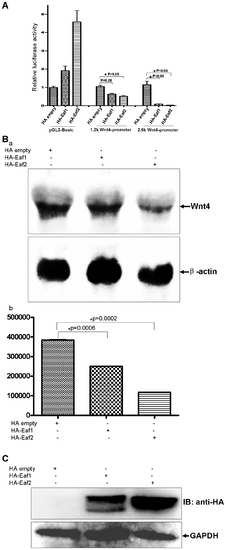
Human Wnt4 expression is suppressed by human EAF1 and EAF2/U19 in a human cell line. (A) 293 cells were transfected with human Wnt4 promoter (1.2 kb and 2.6 kb)-reporter constructs and a control vector or vectors expressing EAF1 or EAF2/U19. Luciferase activity was normalized to Renilla and reported as the mean ± SD. (B) (a) Endogenous Wnt4 expression in 293 cells is suppressed by the over-expression of either EAF1 or EAF2/U19 as revealed by Northern blot analysis. (b) Northern blots were quantitated and then normalized to β-actin mRNA. |
|
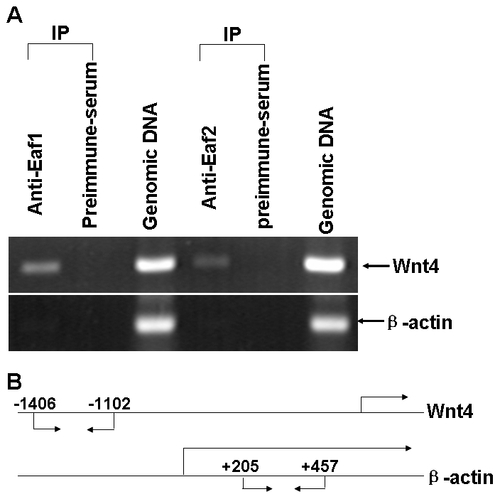
Both human EAF1 and EAF2/U19 bind to the human Wnt4 promoter as revealed by chromatin immunoprecipitaion (ChIP) assays. (A) 293 cells were treated with formaldehyde to create cross-links between EAF1 or EAF2/U19 and chromatin. The chromatin was isolated, sheared, and immunoprecipitated (IP) using polyclonal antibodies against human EAF1 and EAF2/U19, or preimmune serum as control. The presence of chromatin fragments corresponding to the Wnt4 gene or to the β-actin gene promoter was assessed by PCR using gene-specific primers. The gel shows the recovery of Wnt4 and actin gene fragments from the protein-chromatin input on the lane 3 and 6 (from left to right) as well as those recovered after immunoprecipitation with the anti-EAF1 antibody (lane 1), with the anti-EAF2/U19 antibody (lane4) and with the pre-immune serum (land 2 and 5). (B) Schematic diagram depicting the fragment of the EAF1, EAF2/U19 and actin genes that were amplified. The positions of PCR primers used to detect EAF1, EAF2/U19 and actin promoter fragments are indicated by arrows. |