- Title
-
Temporal and spatial requirements of unplugged/MuSK function during zebrafish neuromuscular development
- Authors
- Jing, L., Gordon, L.R., Shtibin, E., and Granato, M.
- Source
- Full text @ PLoS One
|
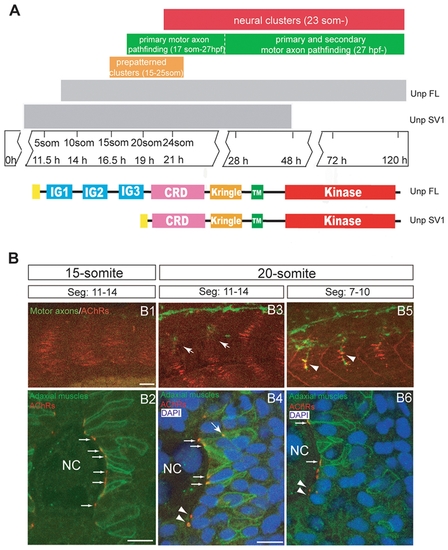
Neuromuscular synaptogenesis in zebrafish. (A) Schematic representation of the different steps during neuromuscular synapse development. unplugged SV1 mRNA is expressed transiently from the tailbud stage to 48 hpf, while unplugged FL mRNA is expressed from the 10-somite stage and continues to be expressed in adult muscle. Bottom: Domain structure of the unplugged FL and SV1 isoforms. (B) Synaptogenesis at the 15- or 20-somite stage. 15-somite (B1) or 20-somite stage (B3 and B4) embryos stained for motor axons (znp-1, green) and AChR clusters (α-BTX, red). In segments 11–14, AChR prepatterned clusters appear at the 15-somite stage before motor neurons have exited the spinal cord (B1); prepatterned clusters coalesce and motor axons (arrows) begin to approach the myotome at the 20-somite stage (B3). In anterior older segments 7–10, motor axons have contacted non-migratory adaxial cells (arrowheads) at the 20-somite stage (B5). (B2, B4, B6) Cross-sectional views of Tg(smyhc1:mcherry-CAAX) embryos, which express mCherry specifically on the membrane of adaxial cells, stained for adaxial muscles (anti-DsRed, green), prepatterned clusters (α-BTX, red) and nuclei (DAPI, blue). The same segments as in (B1, B3, B5) were analyzed. (B2) At the 15-somite stage, adaxial muscles remain adjacent to notochord, and prepatterned clusters are localized at the medial side of the cells (small arrows). (B4) At the 20-somite stage, adaxial muscles have migrated laterally. Some prepatterned clusters co-migrate with the adaxial cells (big arrow). Prepatterned clusters appear on the medial side of fast muscle fibers (arrow heads). Prepatterned clusters remain on the medial side of the non-migratory adaxial cells (small arrows). (B6) In older segments 7–10, adaxial muscles have migrated further away; AChR clusters remain on the medial side of non-migratory adaxial cells (small arrows). AChR clusters appear on the medial side of fast muscle fibers (arrow heads). NC: notochord. Scale bars: 20 μM in B; 10 μM in B2 and B4. |
|
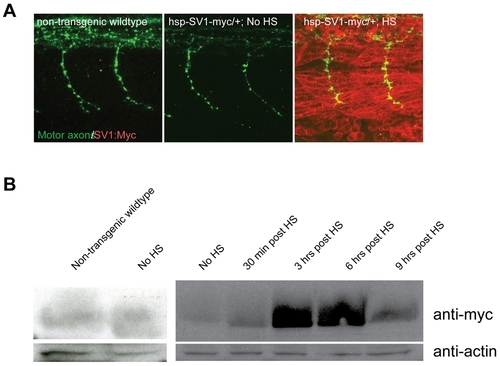
Transgene expression after heat-shock (HS) induction. (A) Lateral views of 26 hpf whole-mount embryos stained with anti znp1 (notor axons, green) and anti myc (red) antibodies. No Myc protein expression is detectable in non-transgenic wildtype and transgenic embryos, but is ubiquitously detectable 10 minutes after a 40-min HS treatment in transgenic embryos. HS treatment was performed at 24hpf for 40 minutes, embryos were fixed and processed at 26 hpf. (B) unplugged/MuSK-myc fusion protein was visualized by western blot with an anti-myc antibody (1:500) in non-transgenic wildtype, transgenic non-heat shocked controls, and 30 minutes, 3 hours, 6 hours and 9 hours after a 40-min HS induction of Tg(hsp70l:unplugged SV1-myc). An anti-actin antibody (1:500) is used as a loading control. Tg(hsp70l:unplugged FL-myc) shows similar results (data not shown). HS treatment was performed at 24hpf for 40 minutes, embryos were fixed and processed at indicated times post heat shock. |
|
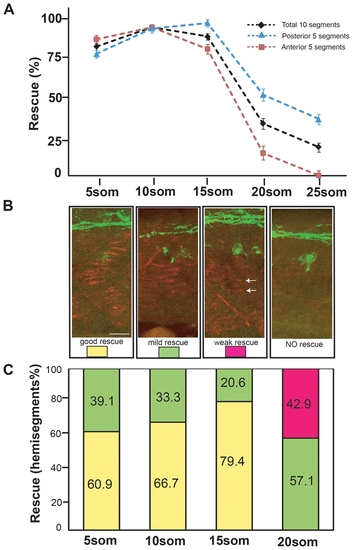
Rescue of motor axon pathfinding and AChR prepatterning by unplugged/MuSK at different time points. (A) Temporal series of Tg(hsp70l:unpFLmyc) induction to rescue motor axon guidance. Embryos were heat-shocked starting at the indicated time points and analyzed at 27 hpf. The induction of Tg(hsp70l:unpFLmyc) expression after the 15-somite stage significantly reduced rescue efficacy (segments 6–15, black; segments 6–10, red; segments 11–15, blue). See Materials and Methods for how rescue efficiency was calculated. 20 hemisegments were analyzed in each embryo, and results from multiple experiments are represented as mean±SEM. (n = 644-1028, average = 744, hemisegments per time point). (B and C) Rescue of AChR prepatterning by Tg(hsp70l:unplugged SV1-myc). Embryos were heat-shocked starting at different time points and examined at the 21-somite stage for prepatterned AChR clusters. Only posterior segments (11-15) were analyzed in each embryo (see also Figure S3). (B) Individual hemisegments were scored as ‘good rescue’ (when AChR clusters were present on most muscles), ‘mild rescue’ (when AChR clusters formed on <50% of all muscles, mostly around non-migratory adaxial cells), or ‘weak rescue’ (with smaller prepatterned clusters formed on a few muscles, see arrows). (C) HS treatment of Tg(hsp70l:unplugged SV1-myc) embryos at the 20-somite stage significantly reduced AChR rescue efficacy. Results were summarized from multiple experiments (n = 7-20, average = 13, hemisegments per time point). Scale bar: 20 μM. |
|
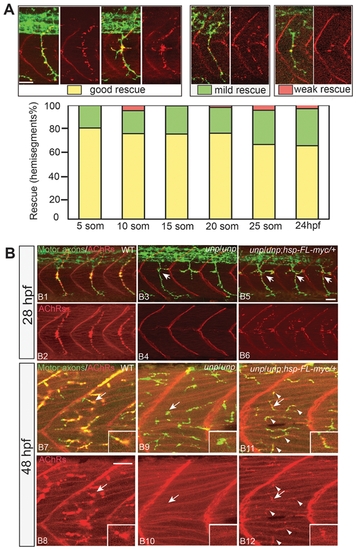
Rescue of neuromuscular synapses by unplugged/MuSK. (A) Rescue of neural synapses at different induction times by Tg(hsp70l:unplugged FL-myc). Embryos were heat-shocked starting at the indicated time points, fixed at 27hpf, and stained for motor axons (znp-1, green) and AChR clusters (α-BTX, red). Individual hemisegments were scored as ‘good rescue’ (when AChR clusters colocalized with presynaptic nerve ending on most muscle fibers, including along ectopic nerve ending), ‘mild rescue’ (when synapses formed on <50% of all muscles), or ‘weak rescue’ (when synapses formed only on non-migratory adaxial cells). 20 hemisegments were scored in each embryo. Results were summarized from two independent experiments (n = 160-380, average = 280, hemisegments per time point). (B) Tg(hsp70l:unplugged FL-myc) rescues neuromuscular synapses at 28 hpf and 48 hpf. Embryos were given a 40-min HS at 28hpf or 48hpf, and examined 30 minutes after the HS treatment. (B1–B6) In transgenic embryos, neuromuscular synapses formed on most muscle fibers and aligned with nerve ending. Arrows point to unplugged-characteristic pathfinding errors, indicating that the HS was too late to rescue axon pathfinding. (B7–B12) The single HS treatment at 48 hpf induced synapses in transgenic embryos (arrowheads in B11 and B12), albeit synapses appear smaller than those in wildtype. Inset in each panel is the enlarged image of the neuromuscular synapse pointed by the arrow. Scale bars: 20 μM. |
|
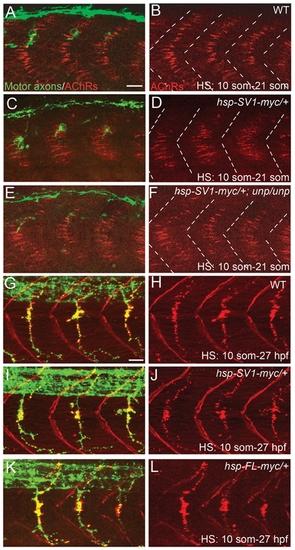
Overexpression of unplugged/MuSK in WT embryos does not perturb synaptogenesis. 21-somite stage (A–F) or 27 hpf (G–L) embryos after HS treatment. (A–F) Wildtype (WT) embryos, Tg(hsp70l:unplugged SV-1myc) embryos, and Tg(hsp70:unplugged SV1-myc); unpluggedbr307/br307 embryos received HS from the 10- to 21-somite stage The AChR prepatterning zone appears slightly expanded in (D) compared to (B and F). White dashed lines mark the boundaries of each segment. (G–L) WT embryos, hsp70l:unplugged SV-myc embryos, (I and J) or hsp70l:unplugged FL-myc embryos (K and L) were heat shocked from the 10-somite stage until 27 hpf. Motor axons and neuromuscular synapses appear normal in the embryos expressing the transgene (I–L). Scale bars: 20 μM. |
|
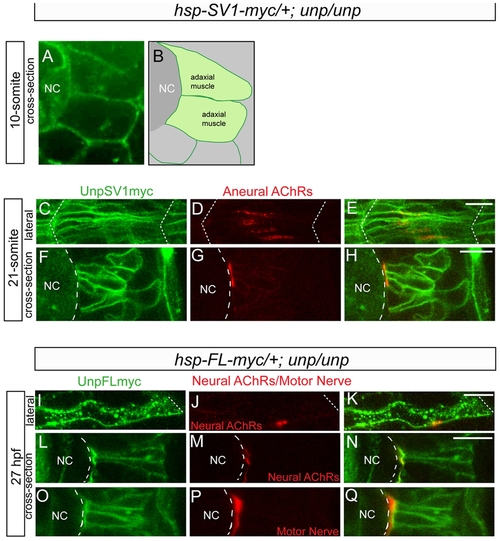
Cellular localization of exogenous unplugged/MuSK protein and AChR clusters. (A) Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 embryos at the 10-somite stage after the HS treatment, stained for anti-Myc Ab (green). Prior to the appearance of AChR clusters Unplugged SV1 protein is distributed evenly on the surface of adaxial muscle cells. (B) Diagram of A. (C–H) Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 embryos at the 21-somite stage after the HS treatment, stained for anti-Myc Ab (green) and AChR clusters (α-BTX, red). Lateral views (C–E) and cross-sectional views (F–H) show that Unplugged SV1 protein is distributed throughout the surface of muscle cells, but prepatterned clusters are localized at the center and the medial side of the cell surface. (I–Q) Tg(hsp70l:unplugged FL-myc); unpluggedbr307/br307 embryos at 27 hpf after HS treatment, stained for anti-Myc Ab (green, I–Q) and AChR clusters (α-BTX, red, J,K,M,N) or znp1(red, P–Q). Unplugged FL protein is expressed throughout the cell membrane as shown in lateral views (I–K), or cross-sectional views, (L–Q). Neural AChR clusters and motor axons are located at the center and the medial side of the cell. Non-migratory adaxial cells at the horizontal myoseptum were imaged. White dashed lines mark the boundaries of the somite in (C–E, I–K) or indicate the position of notochord in (A, B, F–H, L–Q). NC, notochord. Scale bars: 10 μM. |
|
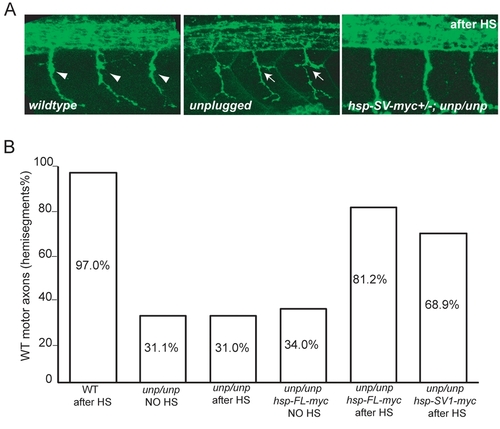
(A) Lateral views of 27-hpf wildtype, unplugged and Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 embryos stained for motor neurons (znp-1). Motor axons in wildtype embryos extend into the ventral myotome after the choice point (arrowheads). unplugged motor axons form lateral branches (arrows), or stall (data now shown) at the choice point. Axonal pathfinding defects were rescued in Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 embryos after the appropriate HS treatment. (B) Quantification of motor axonal phenotypes. Embryos were heat-shocked from the 10-somite stage to 27 hpf. HS treatment did not have obvious effect on motor axon pathfinding in wildtype and unplugged embryos (columns 1–3). In the absence of HS treatment, motor axons remain disrupted in Tg(hsp70l:unplugged FL-myc); unpluggedbr307/br307 (column 4) and Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 (data not shown) embryos. After HS treatment, motor axons were significantly rescued in transgenic embryos (columns 5–6). 20 hemisegments in each embryo were scored. Results are expressed as the average of multiple embryos (n≥20). |
|
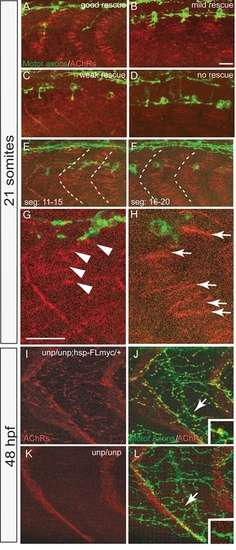
(A-D) Representative images for the AChR prepattern rescue in 21-somite Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 embryos after HS treatments. Embryos were stained for motor neurons (znp-1, green) and AChRs (α-BTX, red). Segments 11-15 were imaged in each embryo. (E-H) Tg(hsp70l:unplugged SV1-myc); unpluggedbr307/br307 embryos were heat-shocked for 40 minutes at the 20-somite stage and examined at the 21-somite stage for motor neurons and AChRs. AChR prepatterning was not well rescued in segments 11-15 (E and G). Posterior segments 16-20 from the same embryo display better rescue of the prepatterning (F and H). (G and H) Enlarged images of the highlighted segments in E and F. Arrow in (H) indicate the large wildtype-like prepatterned clusters. Arrowheads in (G) mark the small punctate clusters. Scale bars: 20 μM. (I-L) Rescue of neural synapses at 48hpf by Tg(hsp70l:unplugged FL-myc). Embryos were heat-shocked for 40 minutes at 48hpf, fixed at 51hpf and stained for motor axons (znp-1, green) and AChR clusters (α-BTX, red). (I and J) Neural synapses induced by heat shock treatment of Tg(hsp70l:unplugged FL-myc); unpluggedbr307/br307 embryos persisted for 3 hours following the heat shock. (K and L) No neural synapses were induced in unpluggedbr307/br307 embryos lacking the transgene. Arrows in I and K point to rescued and non-rescued synapses respectively with insets showing enlarged views. |