- Title
-
Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion
- Authors
- Zhang, J., Piontek, J., Wolburg, H., Piehl, C., Liss, M., Otten, C., Christ, A., Willnow, T.E., Blasig, I.E., and Abdelilah-Seyfried, S.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
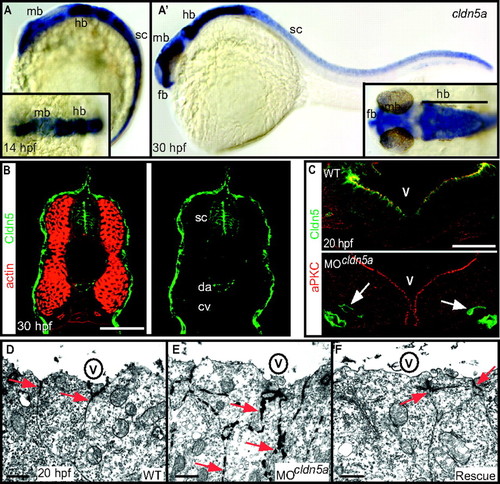
Loss of Claudin5a disrupts the neuroepithelial paracellular barrier function. (A and A′) Whole-mount in situ hybridization of claudin5a (cldn5a) expression at two developmental time points. Dorsal views onto the brain are shown magnified within the insets. (A) Before brain ventricle formation (14 hpf), cldn5a is strongly expressed within the developing central nervous system including the hindbrain (hb) and spinal cord (sc). Weaker expression of cldn5a is found within the dorsal midbrain region (mb). (A′) During ventricle expansion (30 hpf), cldn5a is strongly expressed within the spinal cord and the neuroepithelial ventricular zones of ventral hindbrain and midbrain. There is also strong expression in the forebrain (fb) ventricular zone. (B) Confocal microscopic images of cross-sections through the trunk region. Strong expression of Cldn5 proteins is present within the spinal cord (sc) and the dorsal aorta (da) but not the cardinal vein (cv) at 30 hpf. (C) Injection of MOcldn5a efficiently blocks expression of Cldn5a protein within the apical neuroepithelium lining the brain ventricles (V). Endothelial expression of Cldn5b is not affected in cldn5a morphants (arrows). (D–F) Electron micrographs of neuroepithelial cells covering the cerebral ventricles (V) after intraventricular injection of the electron-dense molecule lanthanum nitrate. In the WT, paracellular clefts are tight for the tracer, which accumulates in a dot-like pattern at the TJ (arrows). Knock-down of cldn5a results in diffusion of lanthanum nitrate into the paracellular space between cells (arrows). In rescue embryos, in which the cldn5a knock-down was rescued by concomitant cldn5a mRNA injection, electron dense material is confined to apico-lateral membranes of neuroepithelial cells similar to the distribution in WT embryos. (Scale bars: B and C, 50 μm; D, 2 μm.) EXPRESSION / LABELING:
PHENOTYPE:
|
|
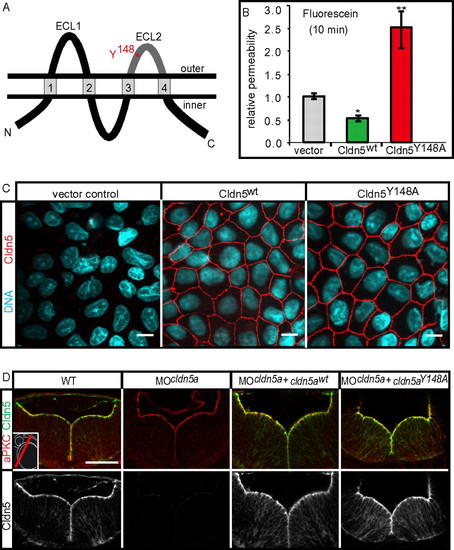
The TJ opening Claudin5aY148A mutant increases TJ permeability. (A) Schematic representation of the four-transmembrane pass TJ protein Claudin5a (Cldn5a). The two extracellular loops (ECL), facing the paracellular space, are involved in claudin-claudin trans interactions. The position of an aromatic residue within ECL2, which is conserved between all classic claudins (17) and which is essential for trans interactions, is highlighted in red. ECL2, which is conserved between all classic claudins (17) and which is essential for trans interactions. (B) Stable transfection of murine Cldn5wt reduces the paracellular permeability of fluorescein (filter culture) compared to the vector control, whereas transfection of the mutant Cldn5Y148A causes an increase of fluorescein permeability. Data represent mean ± SEM, n ≥ 10; *, P < 0.05, **, P < 0.01. (C) Stable transfection of murine Cldn5wt and Cldn5Y148A into MDCK-II cells. The Cldn5 variants are strongly enriched at cell-cell contacts as detected by immunocytochemistry against the N-terminal FLAG-tag. (Scale bars: 10 μm.) (D) Injection of mRNA encoding zebrafish Cldn5awt or Cldn5aY148A into cldn5a morphants results in the correct localization of the proteins at the ventricular surface of the 30 hpf neural tube as detected by confocal microscopy of sectioned immunohistochemical stainings. (Scale bar: 50 μm.) EXPRESSION / LABELING:
|
|
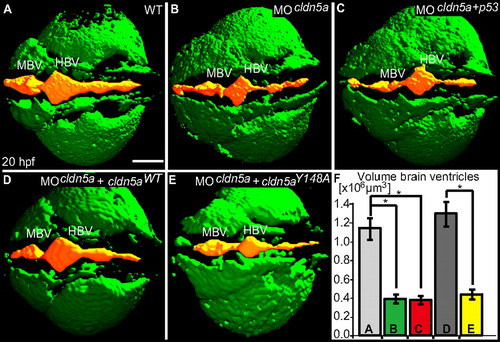
Loss of Claudin5a affects expansion of the brain ventricular lumen at 20 hpf. (A–E) Shown are confocal microscopic Z-scans of in vivo sodium green indicator-labeled embryos. The ventricular lumen is indicated by false-coloring (orange). Indicated are ventricles of the midbrain (MBV) and hindbrain (HBV). (F) Quantifications of ventricular volume were generated from detailed 3D-reconstructions of confocal Z-scan sections for a total of 4–5 embryos per sample group by using Volocity software (unit size is [x106 μm3]). Data represent mean ± SEM, n ≥ 4, *, P = 0.016 in all three cases. (Scale bar: 100 μm.) PHENOTYPE:
|
|
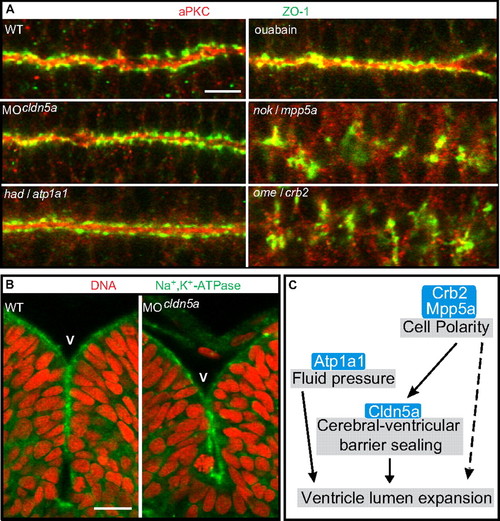
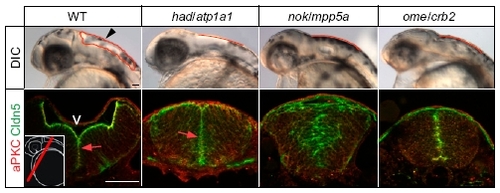
Loss of Claudin5a does not affect neuroepithelial tissue integrity. (A) Shown are apical views of immunohistochemical stainings onto the hindbrain ventricular zone of cell polarity mutants, Cldn5a- or Atp1a1-deficient embryos, or ouabain-treated embryos at 30 hpf. The neuroepithelial localization of Zonula occludens-1 (ZO-1) or aPKC is only affected in the cell polarity mutants nokm520/mpp5a and omem289/crb2. (Scale bar: 20 μm.) (B) Loss of Cldn5a does not affect the neuroepithelial localization of Na+,K+-ATPase which is labeled with the a6F antibody. Shown are confocal microscopic images of sections of immunohistochemical stainings of the hindbrain and ventricle. (Scale bar: 20 μm.) (C) Schematic diagram of developmental/cellular processes contributing to brain ventricle expansion. Our study suggests that the cell polarity regulators Crb2 and Mpp5a are essential for neuroepithelial integrity and maintenance of the TJ. Moreover, Crumbs complex proteins may be directly required for lumen formation (Results and Discussion). Tightness of the TJ is, at least in part, regulated by Cldn5a, which seals the neuroepithelial layer to maintain the fluid pressure, which may depend on the ion pump activity of Atp1a1. Ventricular fluid accumulation drives expansion of brain ventricles and tissue morphogenesis. crumbs2 (crb2); heart and mind (had); membrane protein, palmitoylated 5a (mpp5a); nagie oko (nok); oko meduzy (ome); Ventricle (V). |
|
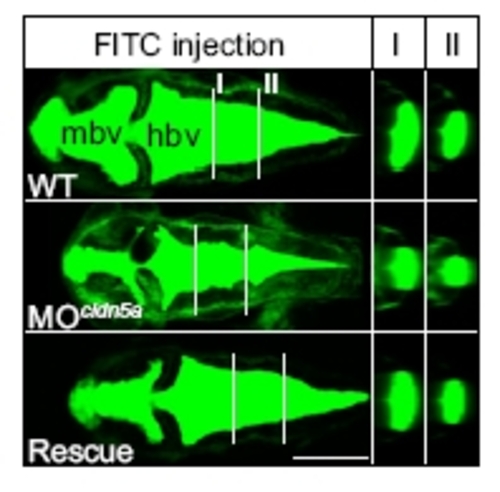
Loss of claudin-5a causes a reduction of brain ventricular volume at 30 h after fertilization. Injection of MOcldn5a significantly reduces the expansion of midbrain (mbv) and hindbrain ventricles (hbv) as assessed by ventricular injection of FITC 70-kDa dextran. White lines indicate different section planes (I and II) of three-dimensional reconstructions of confocal z-stack projections. Coinjection of cldn5a mRNA together with MOcldn5a rescues the brain ventricular expansion defect (Rescue). (Scale bar: 200 μm.) |
|
Phenotypic comparison of brain ventricle expansion defects in different zebrafish ion pump Atp1a1-deficient or cell polarity mutants. Arrowhead indicates location of the hindbrain ventricle in a WT embryo at 30 hpf. Hindbrain ventricular shapes are outlined in red. Sections of immunohistochemical stainings of the brain neuroepithelium show that Cldn5a colocalizes apically together with atypical protein kinase C (aPKC) at the inner ventricular (V) surface at 30 hpf (arrows; Left Inset indicates the cross-sectional plane through the hindbrain region). Localization of Cldn5a and aPKC is affected in nokm520/mpp5a and omem289/crb2 cell polarity mutants. (Scale bar: 50 μm.) |
|
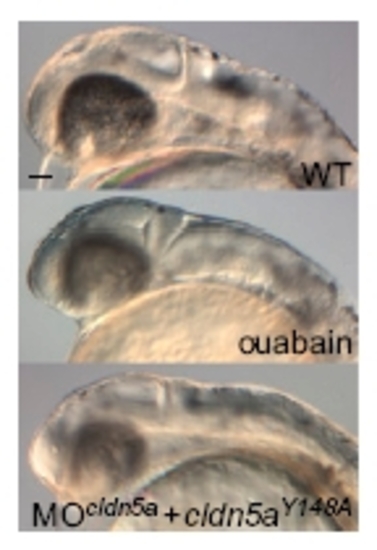
Phenotypic comparison of ouabain-treated and claudin5aY148A mutant embryos during brain ventricular expansion. Phenotypic similarities of brain ventricle expansion defects in ouabain-treated and MOcldn5a and cldn5aY148A mRNA coinjected embryos compared to the WT at 30 hpf. (Scale bar: 50 μm.) |