- Title
-
Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear
- Authors
- Millimaki, B.B., Sweet, E.M., and Riley, B.B.
- Source
- Full text @ Dev. Biol.
|
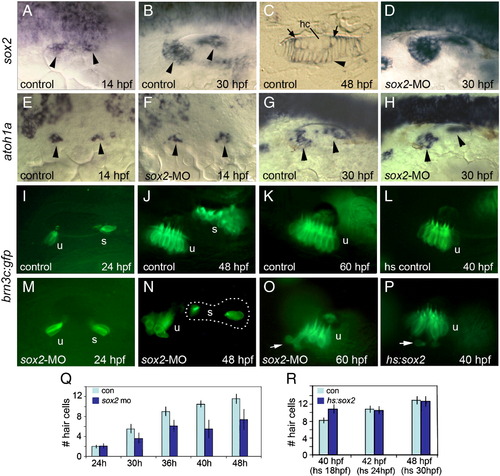
Sox2 is not required for hair cell development. (A–C) sox2 expression in control embryos at 14 hpf (A), 30 hpf (B) and in a cross section of the utricular macula at 48 hpf (C). sox2 expression is lost from mature hair cells (hc) but is still detected in recently formed hair cells (arrows) and all surrounding support cells (arrowhead). (D) sox2 expression at 30 hpf in a sox2 morphant. (E–H) Expression of atoh1a in control embryos (E and G) and sox2 morphants (F and H) at the indicated times. Arrowheads mark macular expression domains. (I–P) brn3c:gfp expression in control embryos at 24 hpf (I), 48 hpf (J) and 60 hpf (K); expression in a control embryo heat shocked at 24 hpf and photographed at 40 hpf (L); expression in sox2 morphants at 24 hpf (M), 48 hpf (N) and 60 hpf (O); and expression in a hs:sox2 transgenic embryo heat shocked at 24 hpf and photographed at 40 hpf (P). Positions of the utricular (u) and saccular (s) maculae are indicated. Note the absence of hair cells in the middle of the saccular macula in the sox2 morphant (N). Arrows in (O) and (P) show hair cells being extruded from the utricular macula. All images show lateral views with anterior to the left and dorsal to the top. (Q) A time course showing the mean number of utricular hair cells in control embryos (con) and sox2 morphants (sox2 mo). Sox2 morphants exhibited a normal number of hair cells at 24 hpf (p = 0.88) but showed significantly fewer hair cells at later time points (p < 0.0001 for each time point). (R) Number of utricular hair cells in control embryos and hs:sox2/+ embryos subjected to heat shock at 18, 24 or 30 hpf, and counted at 40, 42 or 48 hpf, respectively. Transgenic embryos heat shocked at 18 hpf produced significantly more hair cells than normal (p < 0.0004), whereas the number of hair cells was not altered by heat shocking at 24 or 30 hpf (p = 0.78 or 0.73, respectively). Error bars in (Q) and (R) represent standard deviations, with n ≥ 15 for each time point. EXPRESSION / LABELING:
|
|
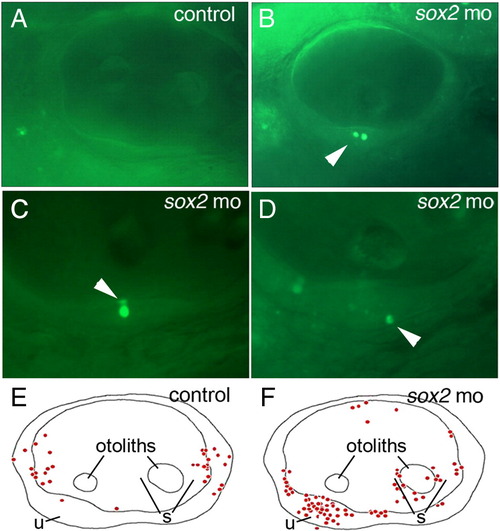
Loss of Sox2 results in macular death. (A–D) AO-labeling of dying cells in a control embryo (A) and sox2 morphants (B–D). Morphants often contained multiple dying cells within sensory epithelia (B), and were observed in apical (C) or basal (D) regions of the maculae (arrowheads). (E and F) Schematic maps depicting the distribution of all AO-positive cells seen in otic vesicles of 33 control embryos (E) or 33 sox2 morphants (F) at 48 hpf. Positions of the utricular macula (u), saccular macula (s) and otoliths are indicated. No AO-positive cells were detected in the lateral wall of the otic vesicle. All images show lateral views with anterior to the left and dorsal to the top. PHENOTYPE:
|
|
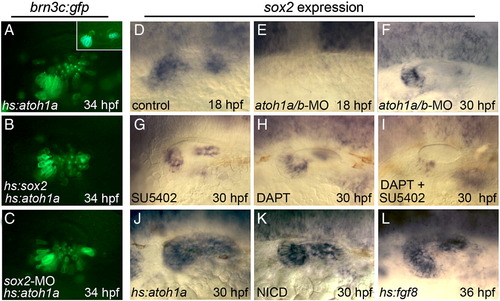
Relationship between Sox2 and upstream regulators of hair cell development. (A–C) Expression of brn3c:gfp in hs:atoh1a/+ transgenic embryos (A and C) and a hs:atoh1a/+;hs:sox2/+ double transgenic embryo (B) heat shocked at 24 hpf and photographed at 34 hpf. The specimen in (C) was also injected with sox2-MO. The inset in (A) shows a heat-shocked brn3c:gfp/+ control embryo at 34 hpf. (D–L) sox2 expression in a control embryo (D), atoh1a/b morphants (E and F), wild-type embryos exposed to SU5402 (G), DAPT (H), or both DAPT and SU5402 (I) beginning at 26 hpf, a hs:atoh1a/+ embryo heat shocked at 24 hpf (J), a hs:gal4/+;UAS-NICD/+ embryo heat shocked at 24 hpf (K), and a hs:fgf8/+ embryo heat shocked at 30 hpf (L). sox2 expression is shown at 30 hpf, except (D) and (E) (18 hpf) and (L) (36 hpf). Expression in control embryos does not change appreciably between 30 and 36 hpf. All images show lateral views with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
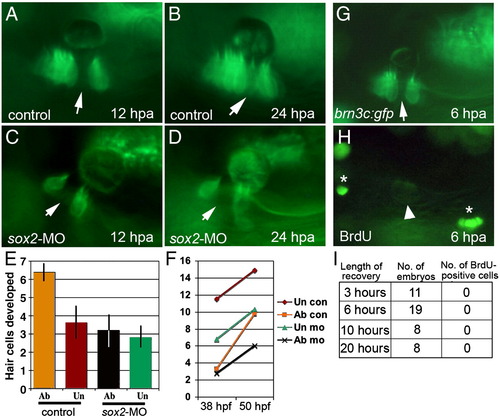
Hair cell regeneration requires sox2 but does not involve cell division. (A–D) brn3c:gfp following ablation in a control embryo (A and B) and a sox2 morphant (C and D). Hair cells were ablated at 48 hpf, and ablated regions (arrows) were still evident at 12 h post-ablation (hpa) (A and C) and 24 hpa (B and D). By 24 hpa, the gap filled in with newly formed hair cells in the control (B) but not in the sox2 morphant (D). (E and F) The number of hair cells produced following wholesale ablation of utricular hair cells. Ablation was conducted at 30 hpf, embryos were allowed to recover, and hair cells were counted at 38 hpf and again at 50 hpf. Typically 2 hair cells were produced during the recovery period. The number of hair cells produced between 38 and 50 hpf (E), and the total number of hair cells (F) are indicated for ablated (ab) and unablated (un) control embryos and sox2-morphants. Each time point shows the mean ± standard error of 3 or 4 experiments, with sample sizes of 19 to 23 embryos. (G–I) BrdU incorporation at various times following ablation initiated at 48 hpf. After 3, 6, 10 or 20 h of recovery, embryos were incubated with BrdU for 3 h and then fixed for processing. A specimen just before fixation at 6 h post-ablation (G) shows that the hair cell gap is still evident (arrow). After processing with anti-BrdU (H), dim GFP fluorescence is still detectable (arrowhead) and shows that no brightly labeled BrdU-positive cells (asterisks) are evident within the macula. |
|
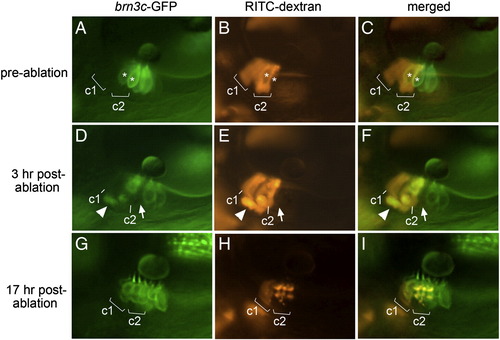
Regeneration occurs through transdifferentiation. (A–C) Lineage-labeled embryo at 48 hpf, just before laser ablation, showing brn3c:gfp-labeled hair cells in the utricular macula (A), two clusters (c1 and c2) of lineage-labeled cells (B) and an overlay showing both labels (C). Most lineage-labeled cells are support cells. Asterisks mark hair cells that were subsequently targeted for ablation. (D–F) The same specimen 3 h post-ablation. A notable gap in the hair cell layer (arrow) marks the position previously occupied by one of the targeted hair cells. Accumulation of lineage label plus GFP beneath the macula appears to show a fragmenting apoptotic hair cell being ejected from the macula (arrowhead). Labeled support cells are still evident in clusters c1 and c2. (G–I) The same specimen 17 h post-ablation. Support cells in cluster c1 are still evident, though fluorescence intensity has decreased as described in Materials and methods. In contrast, lineage label is no longer visible in the support cell layer in cluster c2. Instead, lineage-labeled cells now occupy the hair cell layer and express brn3c:gfp. Much of the lineage label is concentrated in vesicles, as is typical at this stage following laser irradiation (see Materials and methods). All images show lateral views with anterior to the left and dorsal to the top. |
Reprinted from Developmental Biology, 338(2), Millimaki, B.B., Sweet, E.M., and Riley, B.B., Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear, 262-269, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.