- Title
-
FGF-receptor signalling controls neural cell diversity in the zebrafish hindbrain by regulating olig2 and sox9
- Authors
- Esain, V., Postlethwait, J.H., Charnay, P., and Ghislain, J.
- Source
- Full text @ Development
|
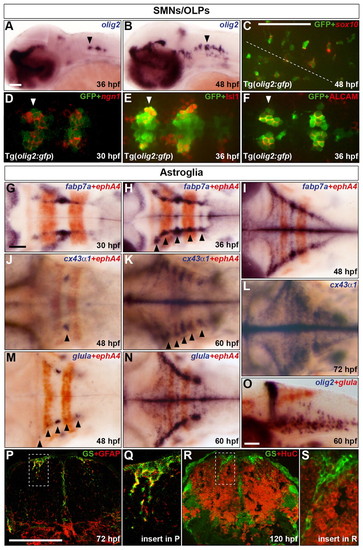
Characterisation of the development of SMNs, OLPs and astroglia in the zebrafish hindbrain. (A,B) Embryos were analysed for olig2 RNA expression at 36 (A) and 48 (B) hpf. (C-F) Co-expression of GFP protein (green) in Tg(olig2:gfp) line and sox10 (C) or ngn1 (D) RNA and Isl1 (E) or ALCAM (F) protein (red) at 48 (C), 30 (D) and 36 (E,F) hpf. In C the position of the midline is indicated by a dashed line. (D-F) Ventromedial r5-7. Arrowheads indicate r5 (A,B,D-F). (G-O) Wild-type embryos were analysed for RNA expression of fabp7a at 30 (G), 36 (H) and 48 (I) hpf, cx43α1 at 48 (J), 60 (K) and 72 (L) hpf and glula at 48 (M) and 60 (N,O) hpf. Embryos were double labelled for ephA4 (red) RNA to mark r3 and r5 (G-K,M,N) or olig2 (blue) (O). Arrowheads indicate dorsolateral r4 (J) and r2-6 (H,K,M). Note that from about 24 hpf, the hindbrain ventricular zone has a characteristic T-shape, where ventral is located ventromedially and dorsal is located dorsolaterally. Embryos are shown with anterior to the left in lateral (A,B,O) or dorsal (C-N) view. (P-S) Transverse sections of the hindbrain at the level of the otic vesicle were analysed for GS (green) and GFAP (red) at 72 hpf (P) or HuC at 120 hpf (R). The framed areas in P and R are shown at higher magnification in Q and S, respectively. Scale bar: 50 μm. |
|
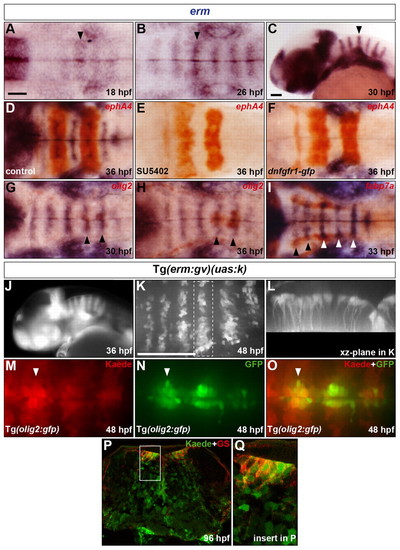
Presence of an FGF-receptor signal in progenitors of hindbrain SMNs, OLPs and GS-positive astroglia. (A-C) Evolution of erm RNA expression in the hindbrain from 18 to 30 hpf. Arrowheads indicate the centre of r4. (D-F) Embryos were analysed at 36 hpf for erm (blue) and ephA4 (red) RNA expression. Wild-type embryos were either treated with DMSO (D) or with SU5402 at 20 hpf (E) and Tg(hsp70l:dnfgfr1-gfp) embryos were heat shocked at 20 hpf (F). (G-I) Embryos were analysed for erm (blue) and olig2 (G,H) or fabp7a (I) (red) RNA expression at 30 (G), 36 (H) and 33 (I) hpf. Arrowheads indicate the centre of r5 and r6 (G,H) or r2-r6 (I). (J) Kaede fluorescence profile in a Tg(erm:gv)(uas:k) embryo at 36 hpf. (K,L) Kaede of a 46 hpf Tg(erm:gv)(uas:k) embryo was completely photoconverted and the embryo was allowed to develop to 48 hpf and was analysed for de novo Kaede fluorescence. Images show half the hindbrain in dorsal (K) and xz-plane of r5 (L). (M-O) Kaede of a 48 hpf Tg(erm:gv)(uas:k)(olig2:gfp) embryo was completely photoconverted and analysed for Kaede (M,O; red) and GFP (N,O; green) fluorescence. The arrowhead indicates the centre of r5. (P,Q) Transverse hindbrain section at the level of the otic vesicle of a Tg(erm:gv)(uas:k) embryo at 96 hpf analysed for Kaede fluorescence (green) and immunolabelling for GS (red). The framed area in P is shown at higher magnification in Q. Embryos are oriented with anterior to the left, in dorsal (A,B,D-I,K,M-O) or lateral (C,J) view. Scale bars: 50 μm. |
|
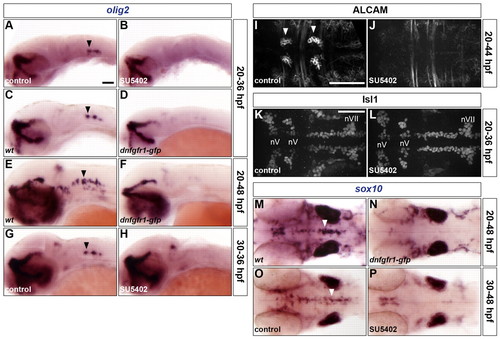
FGF-receptor signalling controls olig2 expression and the generation of both OLPs and SMNs. (A-P) Embryos were analysed for olig2 RNA expression at 36 (A-D,G,H) and 48 hpf (E,F), ALCAM (I,J) and Isl1 (K,L) protein at 44 and 36 hpf, respectively, and sox10 RNA expression at 48 hpf (M-P). Wild-type embryos were either treated with DMSO (A,G,I,K,O) or treated with SU5402 at 20 hpf (B,J,L) or at 30 hpf (H,P) and wild-type (C,E,M) and Tg(hsp70l:dnfgfr1-gfp) (D,F,N) embryos were heat shocked at 20 hpf. Arrowheads indicate ventromedial r5 (A,C,E,G,M,O) and the abducens nuclei in r5 and r6 (I). nV and nVII; branchiomotor nuclei of the V and VII nerves, respectively. All embryos are shown with anterior to the left in lateral (A-H) or dorsal (I-P) view. Scale bars: 50 μm. |
|
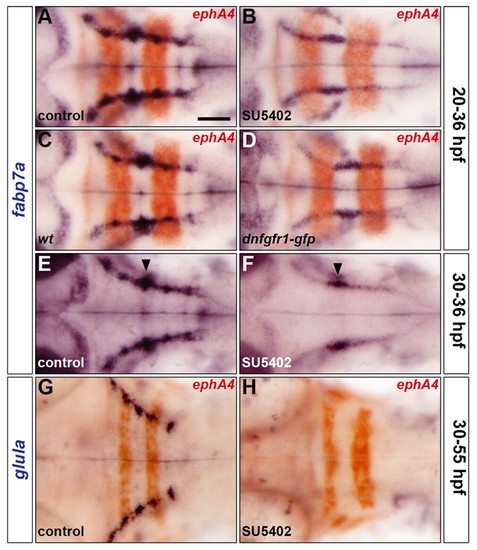
FGF-receptor signalling controls astroglial development. (A-H) Embryos were analysed for fabp7a (A-F) and glula (G,H) RNA expression at 36 hpf and 55 hpf, respectively. Wild-type embryos were either treated with DMSO (A,E,G) or treated with SU5402 at 20 hpf (B) or at 30 hpf (F,H) and wild-type (C) and Tg(hsp70l:dnfgfr1-gfp) (D) embryos were heat shocked at 20 hpf. Embryos were double labelled for ephA4 (red: A-D,G,H) RNA to mark r3 and r5. Arrowheads indicate the centre of r4 (E,F). All embryos are shown in dorsal view with anterior to the left. Scale bar: 50 μm. |
|
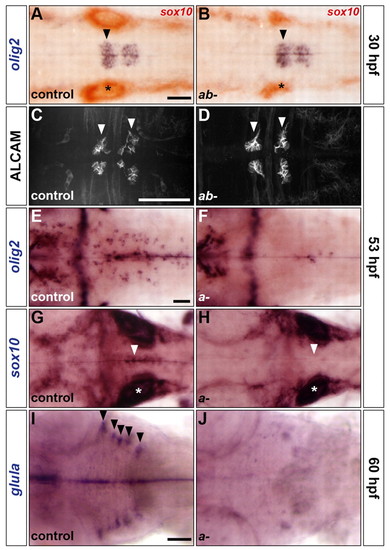
sox9 genes control the development of OLPs and astroglia but not SMNs. (A-J) Analysis of olig2 RNA (A,B,E,F), ALCAM protein (C,D), sox10 RNA (G,H) and glula RNA (I,J) expression in control (A,C,E,G,I), sox9atw37/tw37; sox9bb971/b971 double mutants (B,D) and sox9atw37/tw37 simple mutants (F,H,J) at 30 (A,B), 53 (C-H) and 60 (I,J) hpf. Embryos in A,B were double labelled for sox10 RNA. Control embryos are either homozygous wild type or heterozygous. Arrowheads indicate ventromedial r5 (A,B,G,H), abducens nuclei in r5 and r6 (C,D) and dorsolateral r2-r6 (I). Asterisks, otic vesicle. All embryos are shown in dorsal view with anterior to the left. Scale bars: 50 μm. |
|
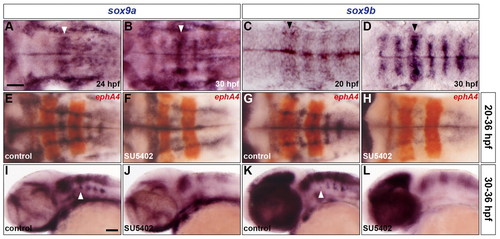
sox9 genes are expressed in rhombomere centres under control of FGF-receptor signalling. (A-D) Expression of sox9a (A,B) and sox9b (C,D) RNA at 24 hpf (A), 30 hpf (B,D) and 20 hpf (C). Arrowheads indicate r4. (E-L) Wild-type embryos were either treated with DMSO (E,G,I,K) or treated with SU5402 at 20 hpf (F,H) and at 30 hpf (J,L) and analysed for sox9a (E,F,I,J) and sox9b (G,H,K,L) RNA expression at 36 hpf. Embryos were double labelled for ephA4 (red: E-H) RNA to mark r3 and r5. Arrowheads indicate ventromedial r4 (I,K). All embryos are shown with anterior to the left in dorsal (A-H) or lateral (I-L) view. Scale bars: 50 μm. |
|
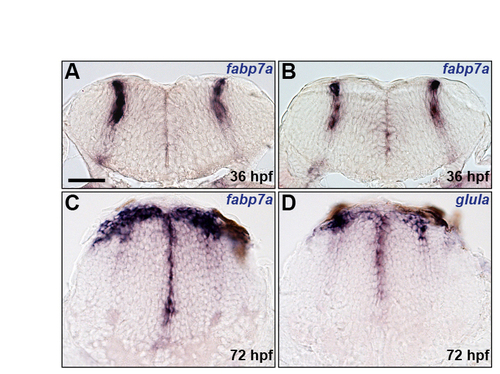
Characterisation of astroglial markers. (A-D) Analysis of fabp7a (A-C) and glula (D) RNA expression at 36 (A,B) and 72 (C,D) hpf. Panels show transverse sections at the centre (A) and boundary (B) regions of r5 and posterior hindbrain (C,D). Scale bar: 50 μm. |
|
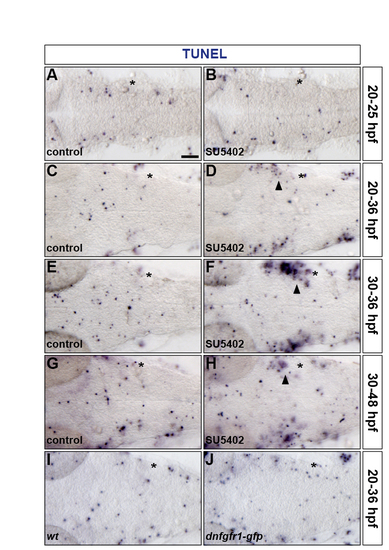
Inhibiting FGF-receptor signalling does not affect hindbrain apoptosis. (A-J) Embryos were analysed for apoptosis by TUNEL labelling at 25 (A,B), 36 (C-F,I,J) and 48 (G,H) hpf. Wild-type embryos were either treated with DMSO (A,C,E,G) or treated with SU5402 at 20 hpf (B,D) and at 30 hpf (F,H) and a wild-type (I) and a Tg(hsp70l:dnfgfr1-gfp) (J) embryo were heat shocked at 20 hpf. Asterisk, otic vesicle. Arrowheads indicate apoptosis in proximity of the otic vesicle outside of the hindbrain. All embryos are shown in dorsal view with anterior to the left. |
|
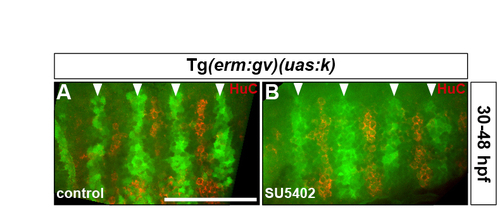
Rhombomere centre cells maintain their progenitor character in the absence of FGF-receptor signalling. (A,B) Tg(erm:gv)(uas:k) embryos were either treated with DMSO (A) or treated with SU5402 at 30 hpf (B) and analysed for Kaede fluorescence (green) and HuC antigen (red) at 48 hpf. Arrowheads indicate the centre of r3-r6. Images show half the hindbrain in dorsal view with anterior to the left. |
|
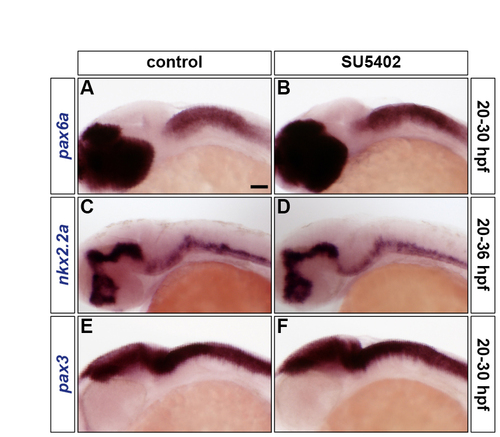
Inhibiting FGF-receptor signalling does not affect DV patterning. (A-F) Wild-type embryos were either treated with DMSO (A,C,E) or treated with SU5402 at 20 hpf (B,D,F) and analysed for pax6a (A,B), nkx2.2a (C,D) and pax3 (E,F) RNA expression at 30 hpf (A,B,E,F) and 36 hpf (C,D). All embryos are shown in lateral view with anterior to the left. |
|
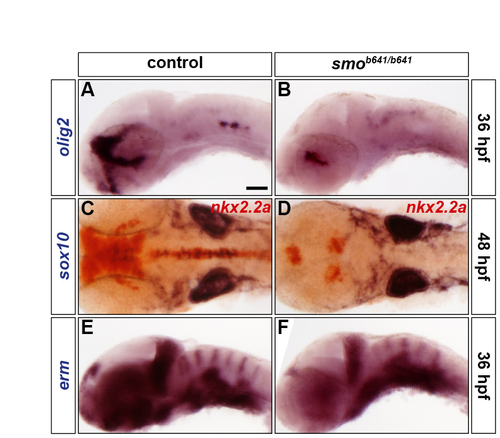
SHH signalling is necessary for olig2 expression and oligodendrocyte formation but does not affect FGF-receptor signalling. (A-F) Analysis of olig2 (A,B), sox10 (blue) and nkx2.2a (red) (C,D), and erm (E,F) RNA expression in control (A,C,E) and smob641/b641 mutants (B,D,F) at 36 hpf (A,B,E,F) and 48 hpf (C,D). Control embryos are either homozygous wild type or smo+/b641 due to the absence of a discriminating phenotype (A,C,E). Embryos are shown in lateral (A,B,E,F) or dorsal (C,D) view with anterior to the left. |
|
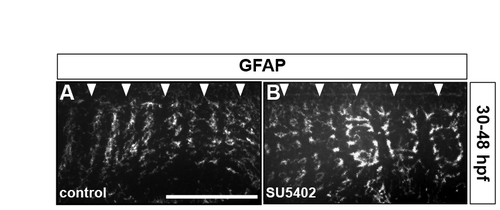
Inhibiting FGF-receptor signalling does not affect GFAP-positive radial glia. Wild-type embryos were either treated with DMSO (A) or treated with SU5402 at 30 hpf (B) and analysed for GFAP antigen at 48 hpf. Arrowheads indicate the centre of r2-r6. Images show half the hindbrain in dorsal view with anterior to the left. |
|
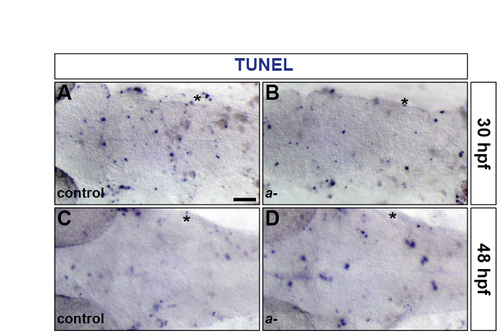
The level of apoptosis is unaffected in the hindbrain of sox9a mutants. (A-D) Control (A,C) and sox9atw37/tw37 mutant (B,D) embryos were analysed for apoptosis by TUNEL labelling at 30 (A,B) and 48 (C,D) hpf. Asterisk, otic vesicle. All embryos are shown in dorsal view with anterior to the left. |