- Title
-
Duplication of fgfr1 Permits Fgf Signaling to Serve as a Target for Selection during Domestication
- Authors
- Rohner, N., Bercsényi, M., Orbán, L., Kolanczyk, M.E., Linke, D., Brand, M., Nüsslein-Volhard, C., and Harris, M.P.
- Source
- Full text @ Curr. Biol.
|
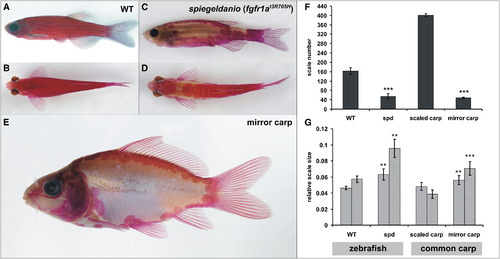
The spiegeldanio Mutant Zebrafish and the Mirror Carp Display Highly Similar Scale Phenotypes (A–E) Alizarin red stain showing the skeleton of wild-type (A and B) and spiegeldanio (spd) (C and D) zebrafish in comparison to the mirror variety of the common carp (E). (A, C, and E) Lateral views. (B and D) Dorsal views. (F and G) Effect of the spd and common carp mirror mutation on the number (F) and size (G) of scales. Error bars represent standard deviation of the mean. **p < 0.005, ***p < 0.0005 versus wild-type (WT). (F) Mean total number of scales from one flank each of three fish from each genotype. (G) Five scales from each of three individual fish for each genotype were used for size analysis (light gray bars indicate anterior-posterior; medium gray bars indicate dorsal-ventral). Size is normalized to total standard length. PHENOTYPE:
|
|
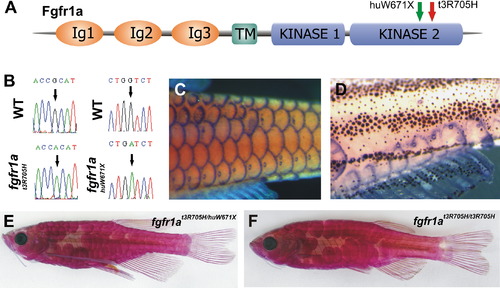
A Mutation in fgfr1a Causes the spd Phenotype (A) Schematic view of predicted fgfr1a structure and site of zebrafish mutations. (B) Nucleotide alteration in fgfr1at3R705H and fgfr1ahuW671X. (C and D) fgfr1a expression in the developing scales and lateral-line organs in wild-type fish (C) and in remaining scales of spd fish (D) (both 10 mm standard length). (E and F) Phenotype of transheterozygous (fgfr1at3R705H/fgfr1ahuW671X) fish (E) showing scale loss similar to spd homozygotes in different genetic backgrounds (F). EXPRESSION / LABELING:
PHENOTYPE:
|
|
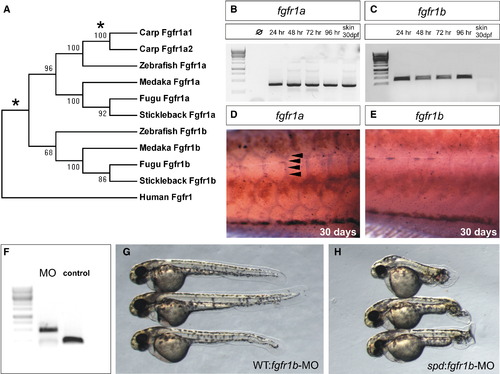
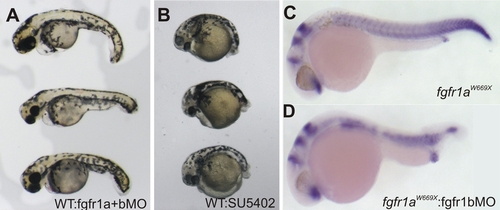
Specific Postembryonic Function of the Zebrafish fgfr1a Gene and Functional Redundancy of fgfr1 Paralogs during Early Embryogenesis (A) Neighbor-joining consensus tree of fgfr1 paralogs in teleosts. Bootstrap values are listed at nodes. Human Fgfr1 is shown as a representative of the sarcopterygian clade. Asterisks denote positions of whole-genome duplication events. (B and C) Analysis of the expression of zebrafish fgfr1a (B) and fgfr1b (C) paralogs during development shows expression through early development of both genes by RT-PCR (35 cycles). Only fgfr1a expression was detected in RNA of skin from fish at 30 days postfertilization (dpf). (D and E) In situ hybridization analysis shows the differential expression of the two paralogs during scale formation. Arrowheads point to regions of expression. (F) Morpholinos (MO) targeting splice site junctions of fgfr1b effectively blocked splicing, leading to inclusion of an intronic sequence assayed by PCR amplification of cDNA. (G and H) Injection of fgfr1b morpholino into wild-type embryos (G) shows little effect, whereas injection into spd embryos (H) results in specific posterior mesenchyme deficiencies of the tail. EXPRESSION / LABELING:
PHENOTYPE:
|
|
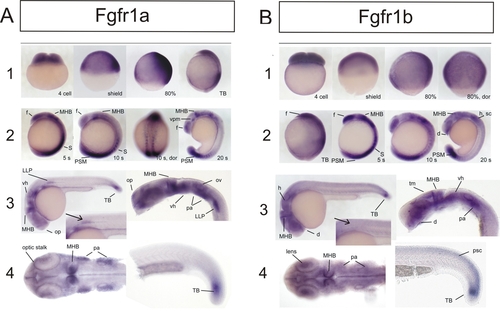
Expression of fgfr1a and fgfr1b during Embryogenesis of the Zebrafish EXPRESSION / LABELING:
|
|
(A, B, and D) Coinjection of fgfr1a and fgfr1b GT-morpholino (0.4 mM and 0.2 mM, respectively) at one-cell stage (A) and Fgfr1 inhibitor (SU5402; 20 μM) treatment of shield stage embryos (B) show effects on the posterior mesoderm similar to injection of fgfr1b GTmorpholino in fgfr1ahuW671X homozygous embryos (D). |