- Title
-
The maternal-effect gene cellular island encodes aurora B kinase and is essential for furrow formation in the early zebrafish embryo
- Authors
- Yabe, T., Ge, X., Lindeman, R., Nair, S., Runke, G., Mullins, M.C., and Pelegri, F.
- Source
- Full text @ PLoS Genet.
|
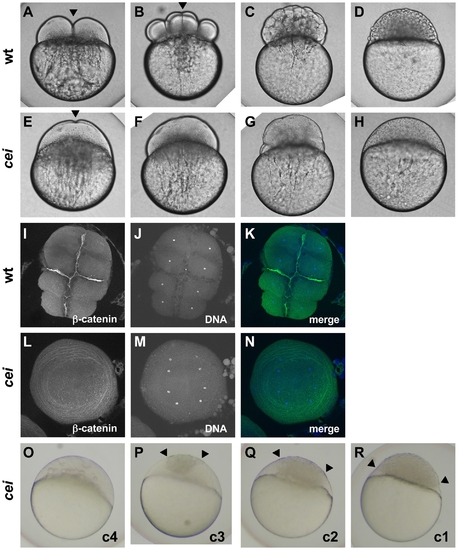
Embryos from cei homozygous females exhibit defects in cytokinesis. Cytokinesis defects in live (A–H, O–R) and fixed (I–N) wild-type (A–D, I–K) and cei mutant (E–H, L–R) embryos. (A–H) Side views of live wild-type (A–D) and maternally mutant cei (E–H) embryos at time points equivalent to (in wild-type embryos) the 2-cell (A,E), 8-cell (B,F), 64-cell (C,G) and 1,000-cell (D,H) stages. Maternally mutant cei embryos exhibit a rudimentary furrow (arrowhead in E) and form syncytial embryos (H). (I–N) Animal views of fixed wild-type (I–K) and maternally mutant cei (L–N) embryos, labeled with an antibody against β-catenin, a component of cell adhesion junctions present in mature furrows (I,L) and the DNA dye DAPI (J,M). Merged images shown in (K,N). (O–R) Side views of live embryos at the 1,000 cell stage, showing the phenotypic range of maternally mutant cei embryos. Embryos exhibit various degrees of aberrant syncytium formation. Images are representative for the categories presented in Table 1: C4 (most severe – O), C3 (P), C2 (Q), C1 (least severe – R). Arrowheads show the limits of a single cellularized region that typically sits atop the syncytial region. |
|
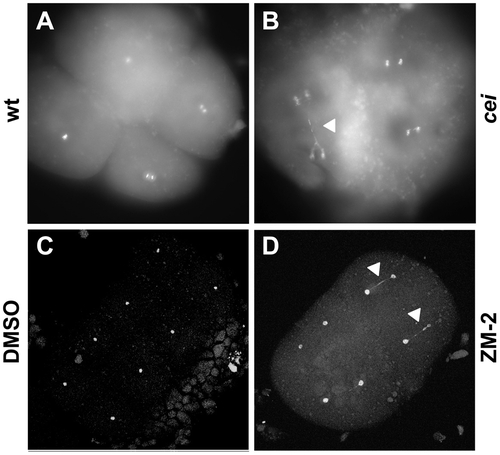
DNA segregation defects in embryos with reduced cei/aurB function. Animal views of fixed embryos labeled with DAPI. (A–B) Wild-type (A) and maternally mutant cei (B) embryos at 60 min p.f. (C–D) Solvent- (C) and ZM2- (D) treated embryos at 75 min p.f. Arrowheads indicate DNA bridges. PHENOTYPE:
|
|
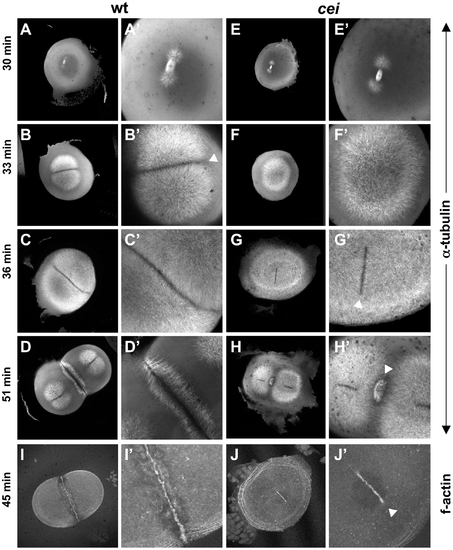
cei mutant embryos have defects in the induction of furrow associated structures. (A–H) Animal views of fixed wild-type (A–D) and mutant (E–H) embryos synchronized by in vitro fertilization and fixed at the indicated time points. Initiation of furrow formation (arrowhead in B′) is delayed in mutant embryos (F′), and furrow associated structures form only in medial region in the mutant (arrowheads in G′,H′). (I,J) Fixed wild-type (I) and mutant (J) embryos labeled with fluorescent phalloidin, showing the formation of a truncated furrow in the center of the blastodisc (arrowhead in J′). PHENOTYPE:
|
|
Defective cytoskeletal dynamics in cei/aurB mutant embryos. (A–G) High magnification images of wild-type (A–C) and cei mutant (D–G) embryos fixed at the indicated time points and labeled with an anti-α-tubulin antibody. Images show the FMA structure, which after formation of the furrow (A) becomes enriched in the distal region of the wild-type embryo (B) and eventually disassembles (C). In maternal cei mutants, the truncated FMA forms in the center of the blastodisc (D) and neither translocates distally nor becomes disassembled (E,F). Arrowheads in (F) indicate FMA remnants corresponding to the second cleavage planes. A rare maternal cei mutant embryo with a weaker phenotype (G) showing that defects in FMA reorganization and disassembly can be observed even when the furrow encompasses the entirety of the blastodisc. |
|
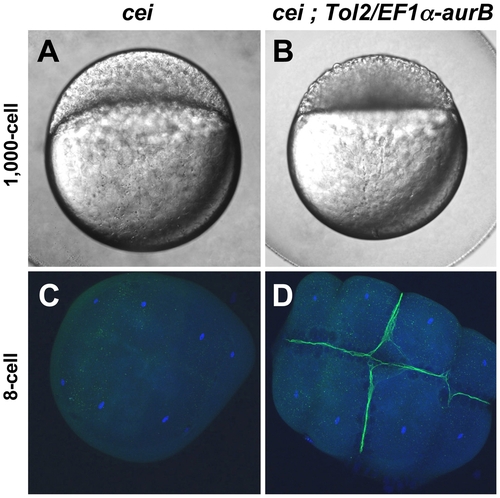
Maternal expression of wild-type aurora B kinase rescues the cytokinesis defects in cei mutant embryos. Embryos from cei/cei homozygous mutant females show a strong syncytial phenotype at the 1,000 cells stage (A) and show defective accumulation of β-catenin in mature furrows at the 8-cell stage (C). Embryos from sibling cei/cei homozygous mutant females which carry transgenic copies of a maternally-expressed wild-type zebrafish aurB gene show significant rescue of the syncytial (B) and β-catenin furrow accumulation phenotype (D). Side views of 1,000-cell stage live embryos (A,B) and animal views of embryos fixed at the 8-cell stage and labeled with an anti-β-catenin antibody (green) and a DNA dye (blue) (C,D). Homozygosity for cei and presence of the transgene were determined by genotyping as in Materials and Methods. |
|
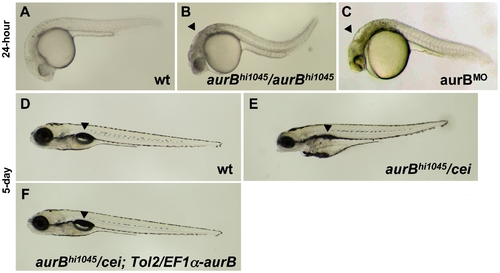
Zygotic cei/aurB function is essential for embryonic development. (A–C) Side views of live 24-hour wild-type (A), hi1045 homozygous (B) and aurB morphant (C) embryos. Arrowheads in (B,C) indicate cell necrosis in the brain. (D–F) Side views of live 5-day wild-type (D) and hi1045/cei transheterozygous (E) embryos, as well as hi1045/cei transheterozygotes containing transgenic copies of the wild-type zebrafish aurB gene (F). Arrowheads indicate the swim bladder, which is inflated in viable embryos (D,F) and indicates inviability when not inflated (E). |
|
Cytokinesis defects in embryos lacking zygotic aurB function. (A–C″) Wild-type (A), aurBhi1045 homozygote (B) and aurB morphant (C) embryos fixed at 24 hours p.f. and labeled to detect β-catenin (green) and DNA (red). (A–C) Overview of the head region. (A′–C′) Optical section through the brain region, showing internal cells. aurBhi1045 homozygotes and aurB morphants show a high frequency of compact nuclei which are typically arranged in pairs (some examples indicated with a white bar at their left flank), consistent with cell death after a failure to undergo proper cytokinesis. (A″–C″) Optical section through a surface layer of the same region, corresponding to the EVL or peridermal layer. In this layer, cells in aurBhi1045 homozygotes and aurB morphants show a high frequency of multilobular nuclei (some examples indicated by asterisks), again suggestive of defects in cytokinesis. Wild-type embryos injected with control MO exhibit normal cellular and nuclear morphologies (not shown), similar to those observed in untreated wild-type embryos (A). (D–E) High magnification images of a wild-type (D) and aurBhi1045 homozygous (E) embryos labeled to detect β-catenin (green), microtubules (red) and DNA (blue). Mutant embryos exhibit closely apposed pairs of nuclei (asterisks) that lack an intervening adhesive membrane. PHENOTYPE:
|
|
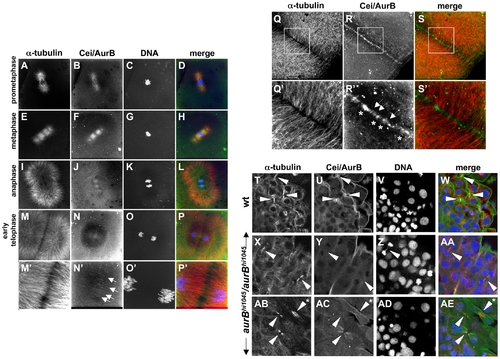
Subcellular localization of Cei/AurB protein. (A–S) Localization of Cei/AurB protein in the early embryo. Animal views of fixed wild-type embryos labeled to detect microtubules, Cei/AurB protein and DNA. (A–P) Series of images detailing the progression of the spindle during the cell cycle as indicated. During prometaphase and metaphase, Cei/AurB protein colocalizes with the forming asters (A–D) and spindles (E–H). During anaphase (I–L), Cei protein remains localized to astral microtubules and the spindle, although localization to the latter appears to subside. During telophase (M–P), Cei/AurB protein transitions to the furrow proper, where it begins to form a ladder-like pattern of short filaments perpendicular to the plane of the furrow (arrows in N′). Panels M′–P′ are 3x magnifications of M–P. (Q–S) During furrow maturation in cytokinesis (shown in this embryo during completion of the first cell division cycle at 38 min p.f.), Cei protein further accumulates along the length of the furrow as punctate aggregates (asterisks in R′) or short filaments perpendicular to the furrow (arrows in R′). These accumulations correspond to sites of microtubule bundling (compare to Q′,S′). Panels Q′–S′ are 3x magnifications of Q–S. All described sites of Cei localization are absent in control labelings using preimmune serum (not shown). (T–AE) Colocalization of Cei/AurB protein to midbodies in fixed 15-somite (16 hours p.f.) embryos. (T–W) Field of cells in a wild-type embryo, showing midbody-like structures (arrowheads in T,U,W). (X–AA, AB–AE) Fields of cells in hi1045 homozygous mutant embryos. Midbody-like structures are present (arrowheads in X,Y,AA,AB,AC,AE) but exhibit reduced levels of Cei/AurB colocalization and often exhibit a splayed, less compact structure (asterisk in AB,AE). In some cases, DNA bridges can also be observed (arrowhead in Z). EXPRESSION / LABELING:
PHENOTYPE:
|
|
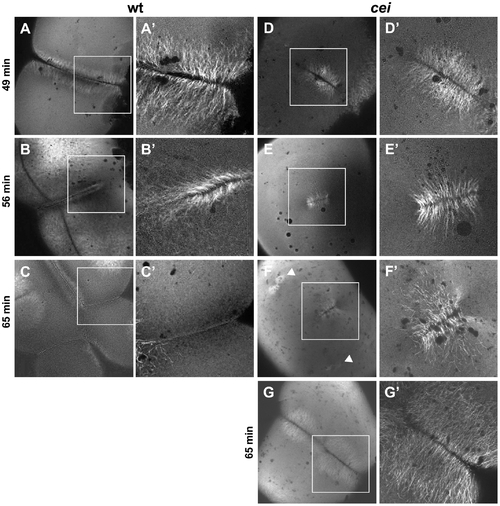
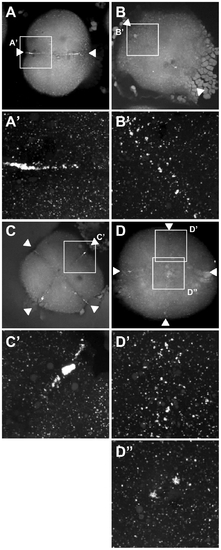
Germ plasm recruitment in cei/aurB mutant embryos. Localization of vasa mRNA by fluorescent in situ hybridization in wild-type embryos fixed at 49 min p.f. (A,B) and 65 min p.f. (C,D). In the wild-type, germ plasm becomes recruited as an elongated rod-like structure in the approximately 2/3 distal regions of the furrow (A′), then localizes as a compact structure at the distal furrow end (C′). Recruitment of germ plasm is reduced or absent in cei mutants (B′, D′). Instead, aggregated germ plasm often becomes recruited to two aggregates in the center of the blastodisc, corresponding to the edges of the truncated furrow-like structure observed in these mutants (D″). Arrowheads flank planes of cleavage. |
|
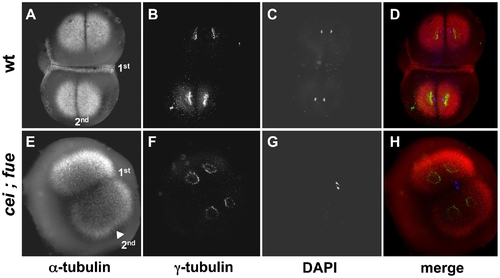
Redundant signals contribute to furrow formation in the early blastomeres. Wild-type (A–D) and cei; fue double mutant (E–H) embryos were fixed at 49 min p.f. and labeled with anti–α-tubulin antibodies (A,E; red in D,H), anti–γ-tubulin antibodies (B,F; green in D,H) and DAPI (C,G; blue in D,H). (A–D) At this stage, wild-type embryos exhibit an FMA along the span of the first cleavage plane (1st). (E–H) cei; fue double mutant embryos lack an FMA in either medial or distal regions of the 1st cleavage plane (compare to Figure 3D,H). Double mutants show the expected delay in furrow induction, characteristic of the cei mutation, in the less mature furrow corresponding to the 2nd cleavage plane (arrowhead in (E); compare to Figure 3B and 3F). Double mutants also exhibit the pronuclear fusion defect characteristic of the fue mutation [31]. Similar results can be observed in embryos until 65 min p.f. (not shown). PHENOTYPE:
|
|
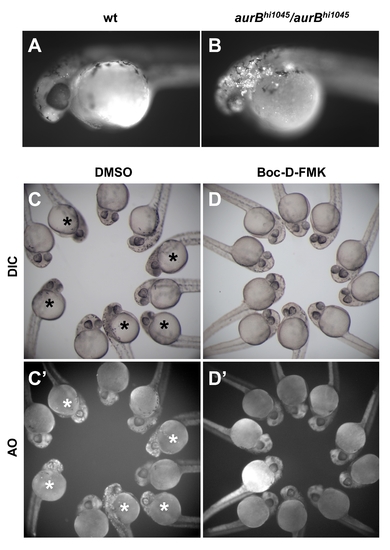
aurBhi1045 homozygotes exhibit an increase in cell apoptosis. (A,B) Exposure to the live cell apoptosis dye acridine orange brightly labels aurBhi1045 homozygotes (B), while control siblings only exhibit background levels of labeling (A). (C–D) Embryos from incrosses between aurBhi1045 heterozytes, 25% of which are expected to be aurBhi1045 homozygotes, were treated with either the caspase inhibitor Boc-D-FMK (D,D′) or control solvent (DMSO; C,C′). Embryos exhibiting the brain necrosis phenotype characteristic of aurBhi1045 homozygotes are indicated with an asterisk. Boc-D-FMK treatment prevents both the brain necrosis visible under standard microscopy (DIC; D, compare to C) and the accumulation of acridine orange labeled cells (AO; D′ compare to C′). |
|
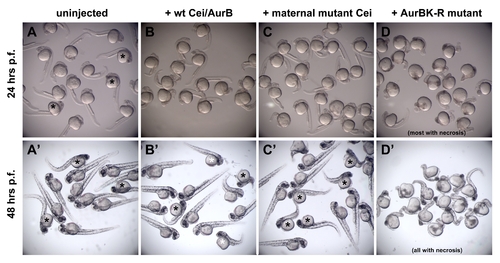
Expression of Cei/AurB products in aurBhi1045 homozygotes. mRNA was injected at the one-cell stage into embryos derived from incrosses of aurBhi1045 heterozygotes, so that an expected 25% of the progeny are aurBhi1045 homozygotes. In (A–C,A′–C′), embryos showing overt signs of necrosis are presumed to be aurBhi1045 homozygotes and are indicated with asterisks. (A–D) At 24 hours p.f., the expected fraction of uninjected embryos begins to show brain necrosis, as indicated by decreased transparency in the head region (A). At this stage, embryos expressing products encoded by the wild-type (B) and maternal-effect mutant (C) alleles show no overt signs of necrosis. Expression of an engineered kinase dead mutant product (AurBK-R; [43]) results in an increased severity of brain necrosis in most embryos (D), showing that this product functions in a dominant negative manner. (A′–D′) Images at 48 hours p.f. of the same groups of embryos as in (A–D). Control uninjected aurBhi1045 homozygotes now show a greater degree of necrosis in the anterior region, as reflected by increased darkening and a reduction of its size (A′). At this stage, aurBhi1045 homozygotes injected with mRNA coding for wild-type (B′) and maternal-effect mutant (C′) Cei/AurB products begin to show brain necrosis, although generally to a lesser extent than uninjected mutant embryos. The degree of phenotypic rescue in embryos expressing wild-type product appears higher than that observed in embryos expressing the maternal-effect mutant product (less affected embryos in B′ are indicated by a white asterisk), which is consistent with the postulated hypomorphic nature of the maternal-effect mutant allele. By this stage, all embryos expressing AurBK-R protein exhibit some degree of necrosis (D′). Results shown are representative of duplicate experiments in embryos from two different clutches. Overexpression of neither maternal-effect mutant nor aurBK-R products (as well as wild-type product) did not have any observable effects on cell division at the cleavage stages (data not shown), possibly because of a temporal delay in protein product accumulation after mRNA injection at the one-cell stage. |
|
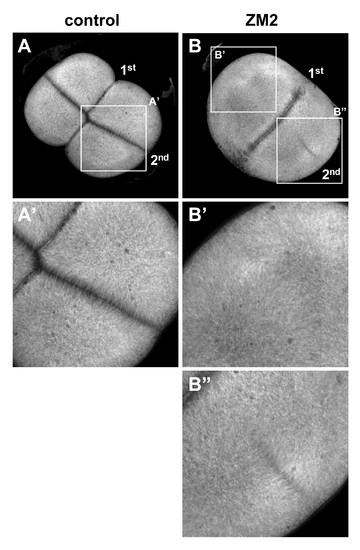
Reduction of aurB function by a small molecule inhibitor phenocopies the cei mutant phenotype. Wild-type embryos treated beginning at 25 min p.f. with either carrier solvent (A) or ZM2 (B), fixed at 55 min p.f., and labeled to detect microtubules. The furrow corresponding to the second cleavage cycle (2nd, which initiates at 45 min p.f.) is either absent (B′) or truncated (B″). Furrow formation during the first cleavage cycle (1st, which initiates at 30 min p.f.) appears unaffected, likely because of a time lag for the effect of the drug. Embryos were synchronized by in vitro fertilization and are shown as animal views. |
|
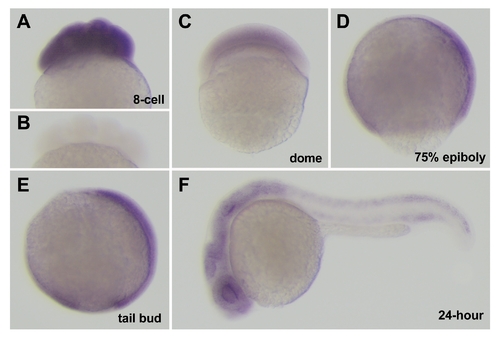
Expression of cei/aurB mRNA during embryogenesis. (A,C–F) Side views of fixed wild-type embryos processed through in situ hybridization using an antisense (A,C–F) and sense (B) cei/aurB probe. Developmental time points are: 8-cell (75 min p.f.), dome (4.3 hours p.f.), 75% epiboly (8 hours p.f.), tail bud (10 hours p.f.), 24-hour (24 hours p.f.). |
|
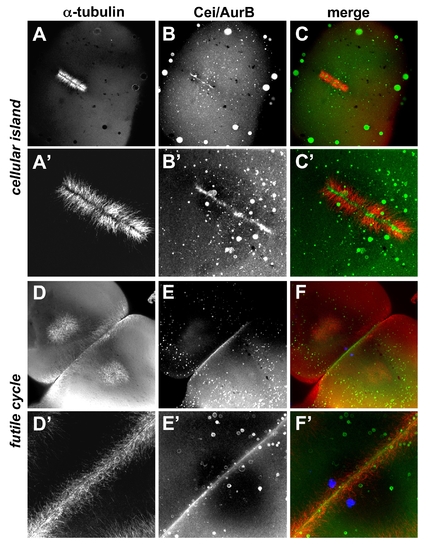
Localization of Cei/AurB protein in futile cycle and maternally mutant cellular island embryos. Animal views of fixed embryos labeled to detect microtubules and Cei/AurB protein. (A–C) In cei mutant embryos, the mutant Cei/AurB protein becomes localized to the ends of FMA tubules that form in the shortened furrow. (D–F) In futile cycle mutant embryos, the FMA forms in an apparently normal manner and Cei/AurB protein is localized throughout the length of the furrow. (A′–C′) and (D′–F′) are higher power images of (A–C) and (D–F), respectively. The DAPI channel is included in (F,F′) to show the unfused pair of pronuclei characteristic of the futile cycle phenotype. |